Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Physical and Chemical Adsorption
Physical
and Chemical Adsorption
Adsorption can be divided into two main categories
– physical and chemical adsorption.
(i) Physical Adsorption
It is the common type of adsorption. The basic
feature of physical adsorption is
that the adsorbate molecules are held at the surface of the adsorbent by weak
van der Waals forces. These are the forces that exist between particles of all
matter. Because of their universal nature, these forces would operate between
any adsorbent and adsorbate pair. Therefore, the physical adsorption is
observed on surface of any solid. Only, the extent of adsorption varies
according to the nature of the adsorbent and adsorbate as discussed earlier.
Physical adsorption is characterized by low enthalpy of adsorption, that is about 10
– 40 kJ mol–1.
Another feature of the physical adsorption of a gas
by a solid is that it is reversible
in nature and an equilibrium is established between the adsorbent and the
adsorbate as discussed earlier. Increase of pressure increases the adsorption
and the release of pressure desorbs the gas. When temperature is increased, the
physical adsorption decreases and when it is lowered, the adsorption increases.
In physical adsorption, several layers of adsorbate are adsorbed one over the
other.
(ii) Chemisorption or Chemical Adsorption
We have seen earlier that some unsaturated
valancies exist on the surface of a solid. Whenever a chemical combination
takes place between the adsorbent and the adsorbate the adsorption becomes very
strong. This type of adsorption caused by forces similar to chemical bonds
between the adsorbent and the adsorbate is called chemisorption or chemical
adsorption.
The enthalpy of chemisorption is as high as that of
chemical bonds (bond enthalpies) and is in the range of 40 – 400 kJ mol–1.
Chemisorption is highly specific and is possible between a specific adsorbent –
adsorbate pair. Like most of the chemical changes it is irreversible. Attempts
to release the adsorbed gas gives the gas and some amount of a definite
compound. For example, oxygen gas is chemisorbed on tungsten. It is released
from the surface of tungsten as a mixture of oxygen and tungsten oxide. Unlike
physical adsorption, chemisorption first increases and then decreases with rise
in temperature [Fig. 17.4 (b)]. This shows that chemisorption has an energy of
activation*. During chemisorption, only one layer of adsorbate molecules is
adsorbed. The main distinctions between physical adsorption and chemisorption
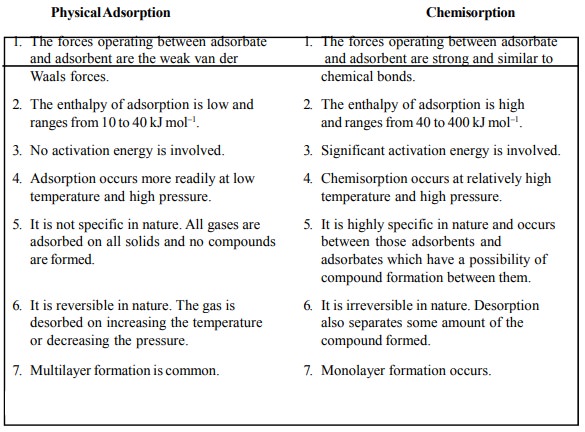
are summarized in Table 17.1.
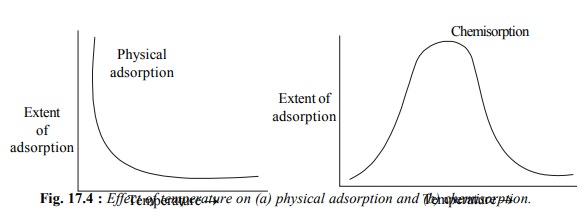
Table 17.1 : Physical Adsorption and Chemisorption
Related Topics