Chapter: Essential Microbiology: Biochemical Principles
Isotopes
Isotopes
Although the number of protons in the nucleus of a given element is always the same, the number of neutrons can vary, to give different forms or isotopes of that element. Carbon-14 (14C) is a naturally occurring but rare isotope of carbon that has eight neu-trons instead of six, hence the atomic mass of 14. Carbon-13 (13C) is a rather more com-mon isotope, making up around 1 per cent of naturally occurring carbon; it has seven neutrons per atomic nucleus. The atomic mass (or atomic weight) of an element is the average of the mass numbers of an element’s different isotopes, taking into account the proportions in which they occur. Carbon-12 is by far the predominant form of the element in nature, but the existence of small amounts of the other forms means that the atomic mass is 12.011. Some isotopes are stable, while others decay spon-taneously, with the release of subatomic particles. The latter are called radioisotopes; 14C is a radioisotope, while the other two forms of carbon are stable isotopes. Radioisotopes have been an extremely useful research tool in a number of areas of molecular biology.
The electrons that orbit around the nucleus do not do so randomly, but are arranged in a series of electron shells, radiating out from the nucleus (Figure 2.1). These layers correspond to different energy levels, with the highest energy levels being located furthest away from the nucleus. Each shell can accommodate a maximum number of electrons, and electrons always fill up the shells starting at the innermost one, that is, the one with the lowest energy level. In our example, carbon has filled the first shell with two electrons, and occupied four of the eight available spaces on the second.
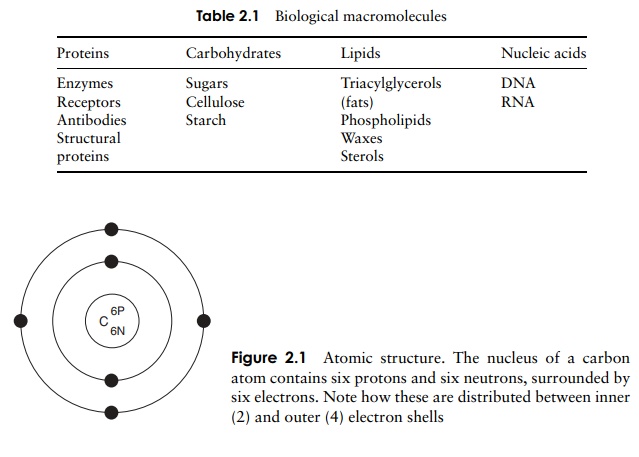
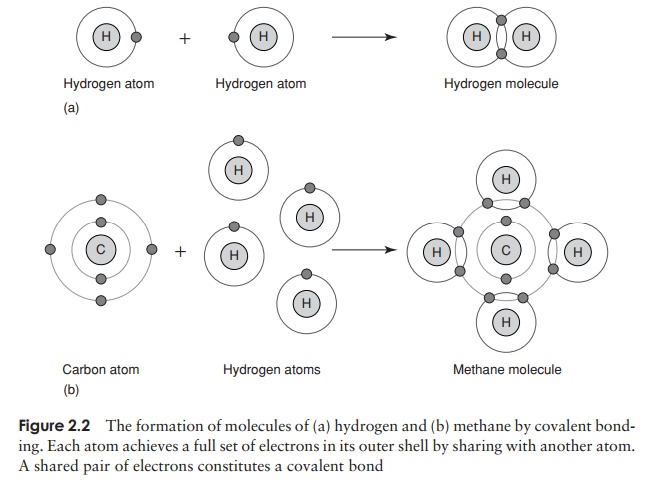
The chemical properties of atoms are determined by the number of electrons in the outermost occupied shell. Neon, one of the ‘noble’ gases, has an atomic number of 10, completely filling the first two shells, and is chemically unreactive or inert. Atoms that do not achieve a similar configuration are unstable, or reactive. Reactions take place between atoms that attempt to achieve stability by attaining a full outer shell. These reactions may involve atoms of the same element or ones of different elements; the result in either case is a molecule or ion . Figure 2.2 shows how atoms combine to form a molecule. A substance made up of molecules containing two or more different elements is called a compound. In each example, the product of the reaction has a full outer electron shell; note that some atoms are donating electrons, while others are accepting them.
The number of unfilled spaces in the outermost electron shell determines the reactivity of an atom. If most of the spaces in the outermost shell are full, or if most are empty, atoms tend to strive for stability by gaining or losing electrons, as shown in Figure 2.3.
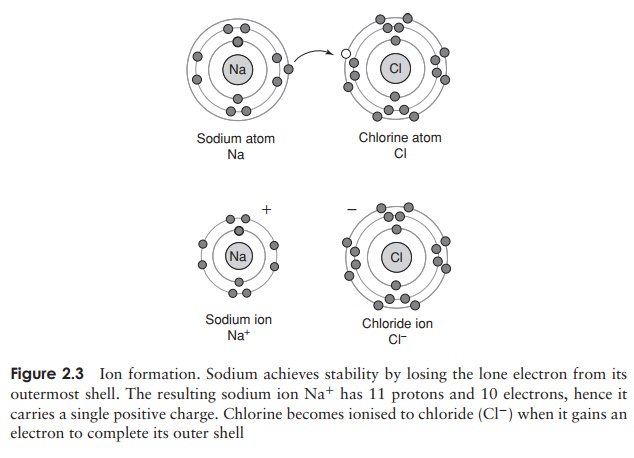
When this happens, an ion is formed, which carries either a positive or negative charge. Positively charged ions are called cations and negatively charged ones anions. The sodium atom for example has 11 electrons, meaning that the inner two electron shells are filled and a lone electron occupies the third shell. If it were to lose this last electron, it would have more protons thanelectrons, and therefore have a net positive charge of one; if this happened, it would become a sodium ion, Na+ (Figure 2.3).
Related Topics