Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Principles of Laboratory Diagnosis of Infectious Diseases
Isolation and Identification of Viruses - Laboratory Diagnosis of Infectious Diseases
Isolation and Identification of Viruses
Cell and Organ Culture
Living cell cultures that can support their replication are the primary means of isolating pathogenic viruses. The cells are derived from a tissue source by outgrowth of cells from a tissue fragment (explant) or by dispersal with proteolytic agents such as trypsin. They are allowed to grow in nutrient media on a glass or plastic surface until a confluent layer one cell thick (monolayer) is achieved. In some circumstances, a tissue fragment with a specialized function (eg, fetal trachea with ciliated epithelial cells) is cultivated in vitro and used for viral detection. This procedure is known as organ culture.
Three basic types of cell culture monolayers are used in diagnostic virology. The primary cell culture, in which all cells have a normal chromosome count (diploid), is de-rived from the initial growth of cells from a tissue source. Redispersal and regrowth produces a secondary cell culture, which usually retains characteristics similar to those of the primary culture (diploid chromosome count and virus susceptibility). Monkey and human embryonic kidney cell cultures are examples of commonly used primary and secondary cell cultures.
Further dispersal and regrowth of secondary cell cultures usually leads to one of two out-comes: the cells eventually die, or they undergo spontaneous transformation, in which the growth characteristics change, the chromosome count varies (haploid or heteroploid), and the susceptibility to virus infection differs from that of the original. These cell cultures have char-acteristics of “immortality”; that is, they can be redispersed and regrown many times (serial cell culture passage). They can also be derived from cancerous tissue cells or produced by ex-posure to mutagenic agents in vitro. Such cultures are commonly called cell lines. A common cell line in diagnostic use is the Hep-2, derived from a human epithelial carcinoma. A third type of culture is often termed a cell strain. This culture consists of diploid cells, commonly fibroblastic, that can be redispersed and regrown a finite number of times; usually 30 to 40 cell culture passages can be made before the strain dies out or spontaneously transforms. Hu-man embryonic tonsil and lung fibroblasts are common cell strains in routine diagnostic use.
Detection of Viral Growth
Viral growth in susceptible cell cultures can be detected in several ways. The most com-mon effect is seen with lytic or cytopathic viruses; as they replicate in cells, they produce alterations in cellular morphology (or cell death) that can be observed directly by light microscopy under low magnification (30 or 100 ). This cytopathic effect (CPE) varies with different viruses in different cell cultures. For example, enteroviruses often produce cell rounding, pleomorphism, and eventual cell death in various culture systems, whereas measles and respiratory syncytial viruses cause fusion of cells to produce multin-ucleated giant cells (syncytia). The microscopic appearance of some normal cell cultures and the CPE produced in them by different viruses are illustrated in Figure 15 – 6.
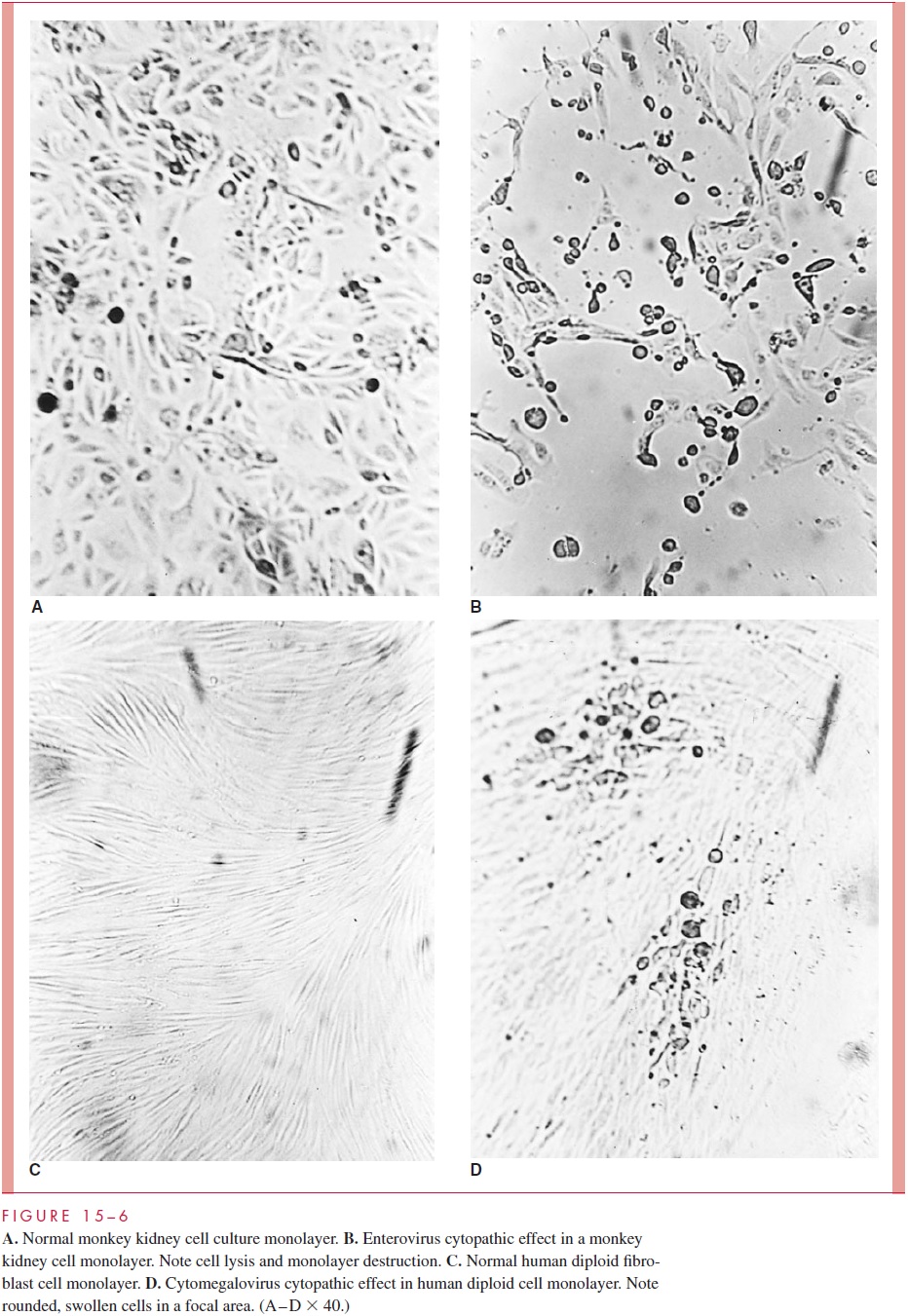
Other viruses may be detected in cell culture by their ability to produce hemagglu-tinins. These hemagglutinins may be present on the infected cell membranes, as well asin the culture media, as a result of release of free, hemagglutinating virions from the cells. Addition of erythrocytes to the infected cell culture results in their adherence to the cell surfaces, a phenomenon known as hemadsorption. Another method of viral detection in cell culture is by interference. In this situation, the virus that infects the susceptible cell culture produces no CPE or hemagglutinin, but can be detected by “challenging” the cell culture with a different virus that normally produces a characteristic CPE. The second, or challenge, virus fails to infect the cell culture because of interference by the first virus, which is thus detected. This method is obviously cumbersome, but has been applied to the detection of rubella virus in certain cell cultures.
For some agents, such as Epstein – Barr virus (EBV) or human immunodeficiency virus (HIV), even more novel approaches may be applied. Both EBV and HIV can replicate in vitro in suspension cultures of normal human lymphocytes such as those derived from neonatal cord blood. Their presence may be determined in several ways; for example, EBV-infected B lymphocytes and HIV-infected T lymphocytes will express virus-specified antigens and viral DNA or RNA, which can be detected with immunologic or genomic probes. In addition, HIV reverse transcriptase can be detected in cell culture by specific as-say methods. Immunologic and nucleic acid probes can also be used to detect virus in clinical specimens or in situations where only incomplete, noninfective virus repli-cation has occurred in vivo or in vitro. An example is the use of in situ cytohybridization, whereby specific labeled nucleic acid probes are used to detect and localize papillomavirus genomes in tissues where neither infectious virus nor its antigens can be detected.
In Vivo Isolation Methods
In vivo methods for isolation are also sometimes necessary. The embryonated hen’s egg is still used for the initial isolation and propagation of influenza A virus. Virus-containing material is inoculated on the appropriate egg membrane, and the egg is incubated to per-mit viral replication and recognition. Animal inoculation is still used for detecting some viruses. The usual animal host for viral isolation is the mouse; suckling mice in the first 48 hours of life are especially susceptible to many viruses. Evidence for viral replication is based on the development of illness, manifested by such signs as paralysis, convul-sions, poor feeding, or death. The nature of the infecting virus can be further elucidated by histologic and immunofluorescent examination of tissues or by detection of specific antibody responses. Many arboviruses and rabies virus are detected in this system.
Viral isolation from a suspect case involves a number of steps. First, the viruses be-lieved most likely to be involved in the illness are considered, and appropriate specimens are collected. Centrifugation or filtration and addition of antimicrobics are frequently re-quired with respiratory or fecal specimens to remove organic matter, cellular debris, bacte-ria, and fungi, which can interfere with viral isolation. The specimens are then inoculated into the appropriate cell culture systems. The time between inoculation and initial detec-tion of viral effects varies; however, for most viruses positive cultures are usually apparent within 5 days of collection. With proper collection methods and application of the diagnos-tic tools discussed later, many infections can even be detected within hours. On the other hand, some viruses may require culture for a month or more before they can be detected.
Viral Identification
On isolation, a virus can usually be tentatively identified to the family or genus level by its cultural characteristics (eg, type of CPE produced). Confirmation and further identification may require enhancement of viral growth to produce adequate quantities for testing. This result may be achieved by inoculation of the original isolate into fresh culture systems (viral passage) to amplify replication of the virus, as well as improve its adaptation to growth in the in vitro system.
Neutralization and Serologic Detection Of the several ways to identify the isolate, themost common is to neutralize its infectivity by mixing it with specific antibody to known viruses before inoculation into cultures. The inhibition of the expected viral effects on the cell culture such as CPE or hemagglutination is then evidence for that virus. As in bacte-riology, demonstration of specific viral antigens is a useful way to identify many agents. Immunofluorescence and enzyme immunoassay (EIA) are the most common methods.
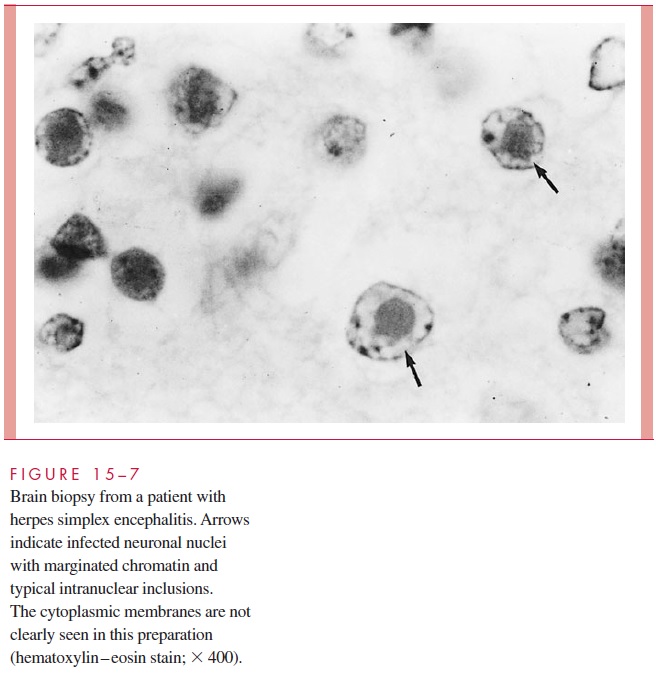
Cytology and Histology In some instances, viruses will produce specific cytologicchanges in infected host tissues that aid in diagnosis. Examples include specific intranu-clear inclusions (herpes, Fig 15 – 7); cytoplasmic inclusions; and cell fusion, which results in multinucleated epithelial giant cells (chickenpox, Fig 15 – 8). Although such findings are useful when seen, their overall diagnostic sensitivity and specificity are usually con-siderably less than those of the other methods discussed.

Electron Microscopy When virions are present in sufficient numbers, they may be further characterized by specific agglutination of viral particles on mixture with type-specific anti-serum. This technique, immune electron microscopy, can be used to identify viral antigens specifically or to detect antibody in serum using viral particles of known antigenicity.
Some viruses (eg, human rotaviruses, hepatitis A and B viruses) grow poorly or not at all in the laboratory culture systems currently available. However, they can be efficientlydetected by immunologic or molecular methods.
Related Topics