Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
How do we choose a buffer?
How do we choose a buffer?
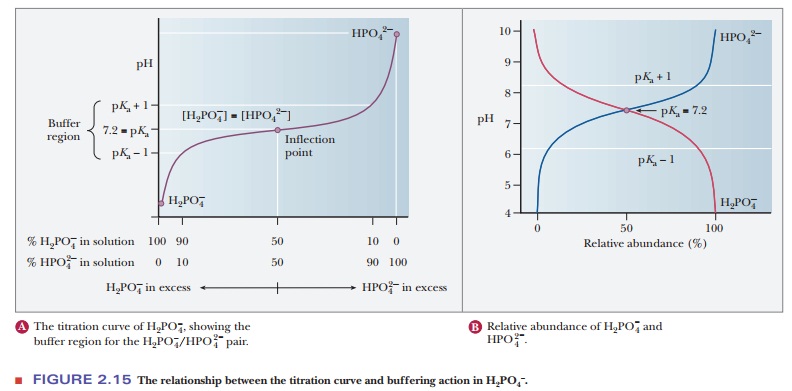
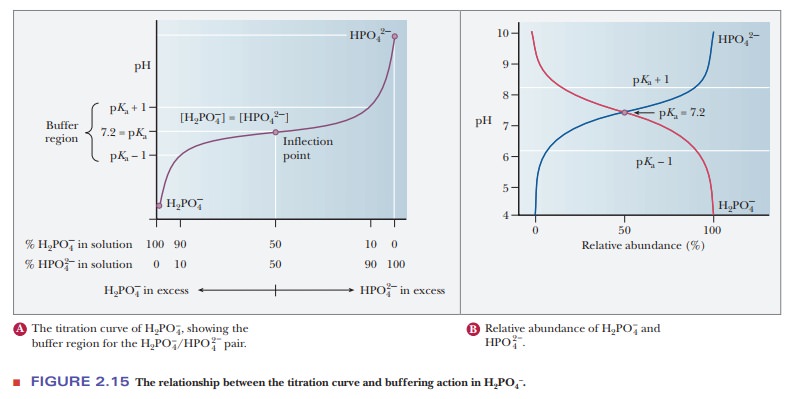
A consideration of titration curves can give insight into how buffers work (Figure 2.15a). The pH of a sample being titrated changes very little in the vicinity of the inflection point of a titration curve. Also, at the inflection point, half the amount of acid originally present has been converted to the conjugate base. The second stage of ionization of phosphoric acid,
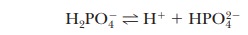
was the basis of the buffer just used as an example. The pH at the inflection point of the titration is 7.20, a value numerically equal to the pKa of the dihydrogen phosphate ion. At this pH, the solution contains equal concentrations of the dihydrogen phosphate ions and monohydrogen phosphate ions, the acid and base forms. Using the Henderson–Hasselbalch equation, we can calculate the ratio of the conjugate base form to the conjugate acid form for any pH when we know the pKa. For example, if we choose a pH of 8.2 for a buffer composed of H2PO4- and HPO42-, we can solve for the ratio
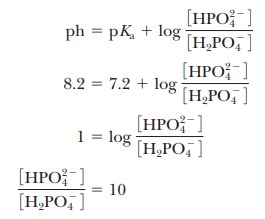
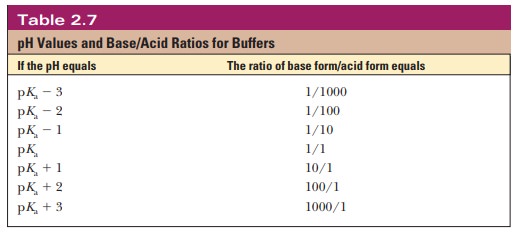
Thus, when the pH is one unit higher than the pKa, the ratio of the conjugate base form to the conjugate acid form is 10. When the pH is two units higher than the pKa, the ratio is 100, and so on. Table 2.7 shows this relationship for several increments of pH value.
A buffer solution can maintain the pH at a relatively constant value because of the presence of appreciable amounts of both the acid and its conjugate base. This condition is met at pH values at or near the pKa of the acid. If OH+ is added, an appreciable amount of the acid form of the buffer is present in solu-tion to react with the added base. If H+ is added, an appreciable amount of the basic form of the buffer also is present to react with the added acid.
The H2PO4/HPO4- pair is suitable as a buffer near pH 7.2, and the CH3COOH/CH3COO- pair is suitable as a buffer near pH 4.76. At pH values below the pKa, the acid form predominates, and at pH values above the pKa, the basic form predominates. The plateau region in a titration curve, where the pH does not change rapidly, covers a pH range extending approximately one pH unit on each side of the pKa. Thus, the buffer is effective within a range of about two pH units (Figure 2.15b).
In many biochemical studies, a strict pH range must be maintained in order for the experiment to be successful. Using our knowledge of the range of an effective buffer compared to its pKa, we can select an appropriate buffer. If we were doing an experiment and needed the pH to be 7.2, we might select the H2PO4/HPO4- pair to be our buffer. If we wanted a pH near 9.0, we would look at tables of buffers to find one with a pKa close to nine. The following Biochemical Connections box goes into greater detail on buffer selection.
The condition that a buffer contains appreciable amounts of both a weak acid and its conjugate base applies both to the ratio of the two forms and to the absolute amount of each present in a given solution. If a buffer solution contained a suitable ratio of acid to base, but very low concentrations of both, it would take very little added acid to use up all of the base form, and vice versa. A buffer solution with low concentrations of both the acid and base forms is said to have a low buffering capacity. A buffer that contains greater amounts of both acid and base has a higher buffering capacity.
Related Topics