Chapter: Essential Microbiology: Biochemical Principles
Higher levels of protein structure
Higher levels of protein structure
The structure of proteins is a good deal more complicated than a just a linear chain of amino acids. A long thin chain is unlikely to be very stable; proteins therefore undergo a process of folding which makes the molecule more stable and compact. The results of this folding are the secondary and tertiary structures of a protein
The secondary structure is due to hydrogen bonding between a carbonyl (-CO) group and an amido (-NH) group of amino acid residues on the peptide backbone (Figure 2.15). The ‘R’ group plays no part in secondary protein structure. Two regular patterns of folding result from this; the α-helix and the β-pleated sheet.
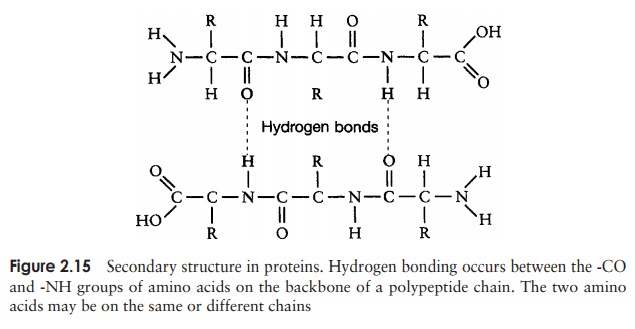
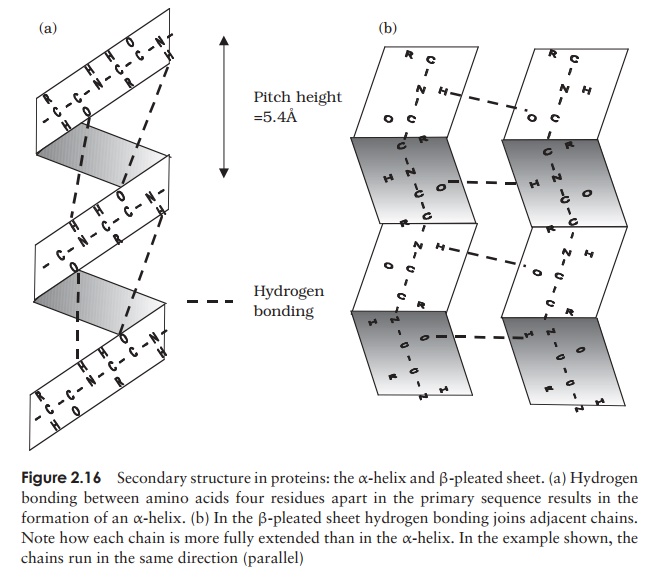
The α-helix occurs when hydrogen bonding takes place between amino acids close together in the primary structure. A stable helix is formed by the -NH group of an amino acid bonding to the -CO group of the amino acid four residues further along the chain (Figure 2.16a). This causes the chain to twist into the characteristic he-lical shape. One turn of the helix occurs every 3.6 amino acid residues, and results in a rise of 5.4 A (0.54 nm); this is called the pitch height of the helix. The ability to form a helix like this is dependent on the component amino acids; if there are too many with large R-groups, or R-groups carrying the same charge, a stable helix will not be formed. Because of its rigid structure, proline (Figure 2.13) cannot be accommodated in anα-helix. Naturally occurring α-helices are always right-handed, that is, the chain of amino acids coils round the central axis in a clockwise direction. This is a much more stable configuration than a left-handed helix, due to the fact that there is less steric hindrance (overlapping of electron clouds) between the R-groups and the C==O group on the peptide backbone. Note that if proteins were made up of the D-form of amino acids, we would have the reverse situation, with a left-handed form favoured. In the β-pleated sheet, the hydrogen bonding occurs between amino acids either on separate polypeptide chains or on residues far apart in the primary structure (Figure 2.16b). The chains in a β-pleated sheet are fully extended, with 3.5 A (0.35 nm) between adjacen amino acid residues (c.f. α-helix, 1.5 A). When two or more of these chains lie next to each other, extensive hydrogen bonding occurs between the chains. Adjacent strands in a β-pleated sheet can either run in the same direction (e.g. N→C), giving rise to a par-allel β-pleated sheet, or in opposite directions (antiparallel β-pleated sheet, as shown in Figure 2.16b).
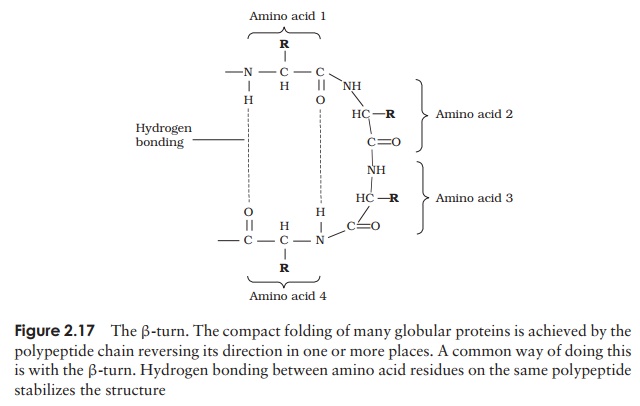
A common structural element in the secondary structure of proteins is the β-turn. This occurs when a chain doubles back on itself, such as in an antiparallel β-pleated sheet. The -CO group of one amino acid is hydrogen bonded to the -NH group of theresidue three further along the chain. Frequently, it is called a hairpin turn, for obvious reasons (Figure 2.17). Numerous changes in direction of the polypeptide chains result in a compact, globular shape to the molecule.
Typically about 50 per cent of a protein’s secondary structure will have an irregular form. Although this is often referred to as random coiling, it is only random in the sense that there is no regular pattern; it still contributes towards the stability of the molecule. The proportions and combinations in which α-helix, β-pleated sheet and random coiling occur varies from one protein to another. Keratin, a structural protein found in skin, horn and feathers, is an example of a protein entirely made up of α-helix, whilst the lectin (sugar-binding protein) concanavalin A is mostly made up of β-pleated sheets.
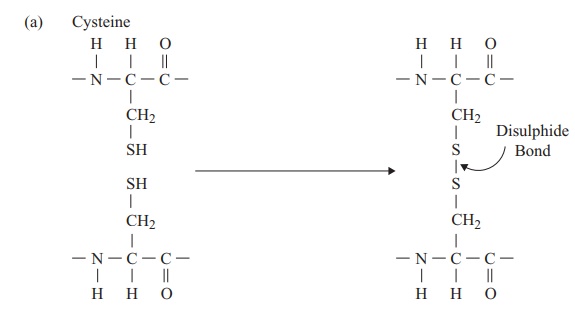
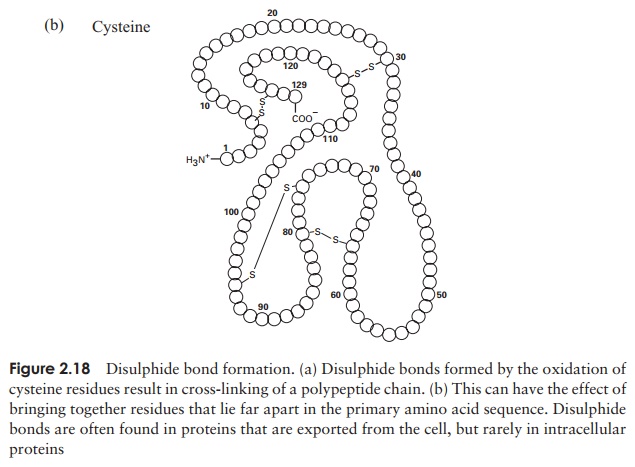
The tertiary structure of a protein is due to interactions between side chains, that is, R-groups of amino acid residues, resulting in the folding of the molecule to produce a thermodynamically more favourable structure. The structure is formed by a variety of weak, non-covalent forces; these include hydrogen bonding, ionic bonds, hydrophobic interactions, and Van der Waals forces. The strength of these forces diminishes with distance, therefore the formation of a compact structure is encouraged. In addition, the -SH groups on separate cysteine residues can form a covalent -S—S- linkage. This is known as a disulphide bridge and may have the effect of bringing together two cysteine residues that were far apart in the primary sequence (Figure 2.18).
In globular proteins, the R-groups are distributed according to their polarities; non-polar residues such as valine and leucine nearly always occur on the inside, away from the aqueous phase, while charged, polar residues including glutamic acid and histidinegenerally occur at the surface, in contact with the water.
The protein can be denatured by heating or treatment with certain chemicals; this causes the tertiary structure to break down and the molecule to unfold, resulting in a loss of the protein’s biological properties. Cooling, or removal of the chemical agents, will lead to a restora-tion of both the tertiary structure and biological activity, showing that both are entirely dependent on the primary sequence of amino acids.
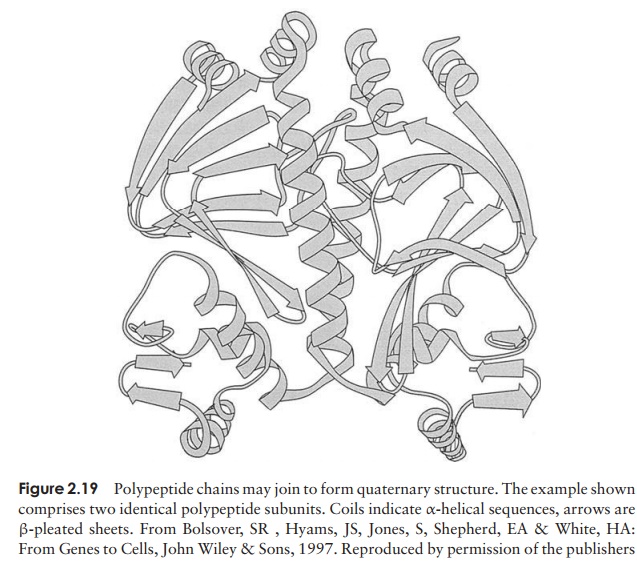
Even the tertiary structure is not always the last levelof organisation of a protein, because some are made up of two or more polypeptide chains, each with its own secondary and tertiary structure, combined together to give the quaternary structure (Figure 2.19). These chains may be identical or different, dependingon the protein. Like the tertiary structure, non-covalent forces between R-groups are responsible, the difference being that this time they link amino acid residues on separate chains rather than on the same one.
Such proteins lose their functional properties if dissociated into their constituent units; the quaternary joining is essential for their activity. Phosphorylase A, an enzyme involved in carbohydrate metabolism, is an example of a protein with a quaternary structure. It has four subunits, which have no catalytic activity unless joined together as a tetramer.
Although all proteins are polymers of amino acids existing in various levels of structural complexity as we have seen above, some have additional, non-amino acid components. They may be organic, such as sugars (gly-coproteins) or lipids (lipoproteins) or inorganic, includ-ing metals (metalloproteins) or phosphate groups (phos-phoproteins). These components, which form an integral part of the protein’s structure, are called prosthetic groups.
Related Topics