Chapter: Biochemistry: The Importance of Energy Changes and Electron Transfer in Metabolism
Coenzyme A in Activation of Metabolic Pathways
Coenzyme A in Activation of
Metabolic Pathways
The metabolic oxidation of glucose does not take place in one step.
The anaerobic breakdown of glucose requires many steps, and the complete
aerobic oxidation of glucose to carbon dioxide and water has still more steps.
One of the most important points about the multistep nature of all metabolic
processes, including the oxidation of glucose, is that the many stages allow
for efÞcient production and use of energy. The electrons produced by the
oxidation of glucose are passed along to oxygen, the ultimate electron
acceptor, by intermediate electron acceptors. Many of the intermediate stages
of the oxidation of glucose are coupled to ATP production by phosphorylation of
ADP.
Why is coenzyme A such a good example of activation?
A step frequently encountered in metabolism is the process of activation. In a reaction of this sort,
a metabolite (a component of a metabolic pathway) is bonded to some other
molecule, such as a coenzyme, and the free-energy change for breaking this new
bond is negative. In other words, the next reaction in the metabolic pathway is
exergonic. For example, if substance A is the metabolite and reacts with
substance B to give AB, the following series of reactions might take place:
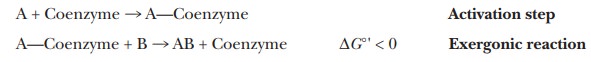
The formation of a more reactive substance in this fashion is
called activation. There are many examples of activation in metabolic
processes. We can dis-cuss one of the most useful examples now. It involves
forming a covalent bond to a compound known as coenzyme A (CoA).
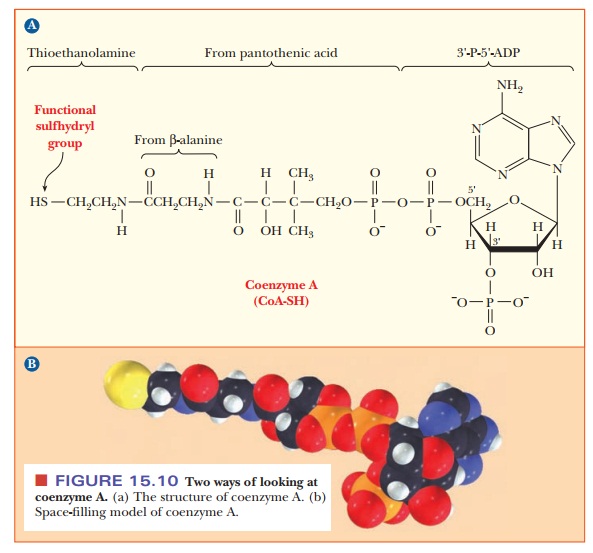
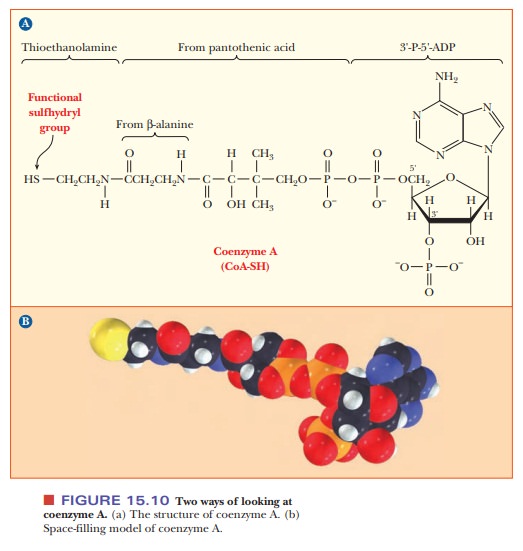
The structure of CoA is complex. It consists of several smaller
components linked together covalently (Figure 15.10). One part is 3'-P-5'-ADP,
a derivative of adenosine with phosphate groups esterified to the sugar, as
shown in the structure. Another part is derived from the vitamin pantothenic
acid, and the part of the molecule involved in activation reactions contains a
thiol group. In fact, coenzyme A is frequently written as CoA-SH to emphasize
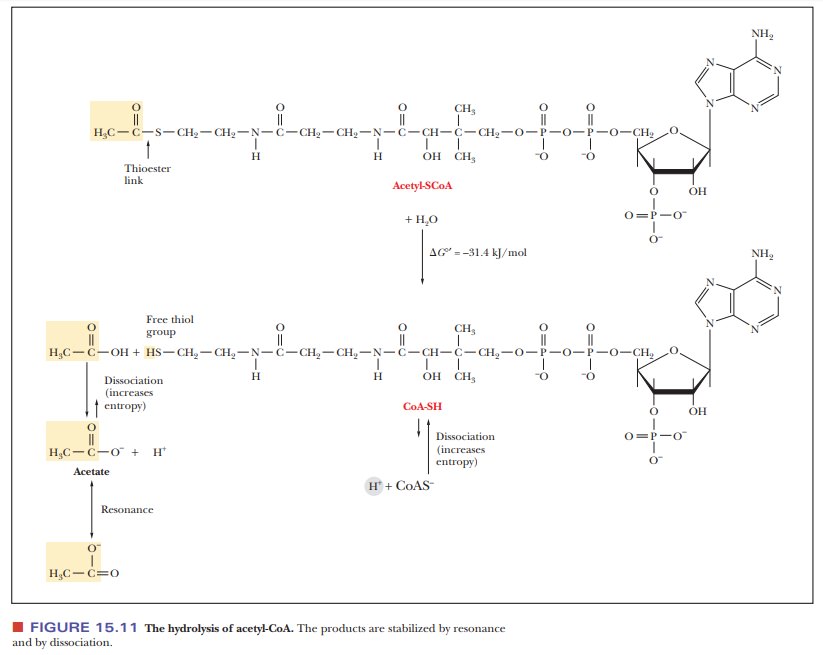
that the thiol group is the reactive portion of the molecule. For example, carboxylic
acids form thioester linkages with CoA-SH. The metabolically active form of a
carboxylic acid is the corresponding acyl-CoA thioester, in which the thioester
linkage is a high-energy bond (Figure 15.11). Thioesters are high-energy
com-pounds because of the possible dissociation of the products after
hydrolysis and resonance structures of the products. For example, when
acetyl-CoA is hydrolyzed, the ÑSH at the end of the molecule can dissociate
slightly to form H+ and CoA-S-. The
acetate released by the hydrolysis is stabilized by resonance. Acetyl-CoA is a
particularly important metabolic intermediate; other acyl-CoA species figure
prominently in lipid metabolism.
The important coenzymes we have met ÑNAD+, NADP+, FAD,
and coenzyme AÑshare an important structural feature: all contain ADP. In NADP+, there
is an additional phosphate group at the 2' position of the ribose group of ADP.
In CoA, the additional phosphate group is at the 3' position.
Like catabolism, anabolism proceeds in stages. Unlike catabolism,
which releases energy, anabolism requires energy. The ATP produced by
catabolism is hydrolyzed to release the needed energy. Reactions in which
metabolites are reduced are part of anabolism; they require reducing agents,
such as NADH, NADPH, and FADH2, all of which are the
reduced forms of coenzymes In their oxidized forms, these coenzymes serve as
the intermediate oxidizing agents needed in catabolism. In their reduced form,
the same coenzymes provide the Òreducing powerÓ needed for the anabolic
processes of biosynthesis; in this case, the coenzymes act as reducing agents.
We are now in a position to expand on our earlier statements about the natures of anabolism and catabolism. Figure 15.12 is an outline of metabolic pathways that explicitly points out two important features of metabolism: the role of electron transfer and the role of ATP in the release and utilization of energy.
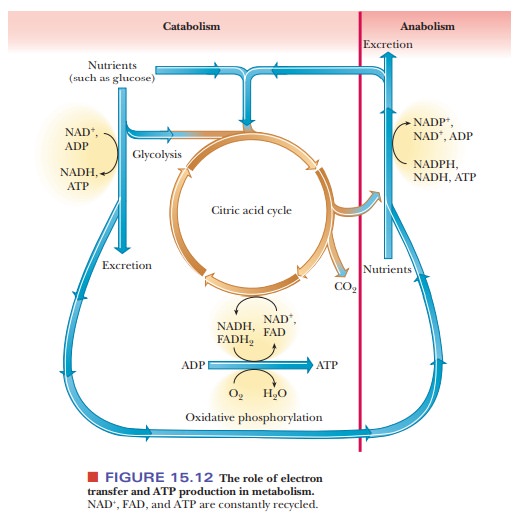
Even though this outline is more extended than the one in
Figure 15.1, it is still very general. The more important specific pathways
have been studied in detail, and some are still the subjects of active
research.
Summary
Metabolic pathways proceed in many stages, allowing for efficient
use of energy.
Many coenzymes, particularly coenzyme A (CoA) play a crucial role
in metabolism.
Related Topics