Chapter: Biochemistry: The Importance of Energy Changes and Electron Transfer in Metabolism
Living Things Need Energy-How Do They Use It?
Living Things Need Energy-How Do They Use It?
Gibbs free energy, ∆G, is perhaps the most suitable way to measure energy changes in living systems because it measures theenergy available to do work at constant temperature and pressure, whichdescribes the living state. Even cold-blooded organisms are at constant temperature and pressure at any given point in time; any temperature and pressure changes are slow enough not to affect measurements of ∆G.
Spontaneity and Reversibility
The concept of spontaneity can be confusing, but it merely means that a reaction can occur without added energy. This is similar to water held behind a dam at the top of a hill, which has the potential energy to ßow downhill, but it does not do so unless someone opens the dam. Because water ßows only downhill, that is the direction with a negative value of the free-energy change (-∆G); pumping water uphill is nonspontaneous (requires energy) and has a positive value of the free-energy change (+∆G). If the free-energy change is only 1 kcal mol-1 (about 4 kJ mol-1) in either direction, then the reaction is considered freely reversible. The reaction can readily go in either direction. If one adds reactants or removes products,
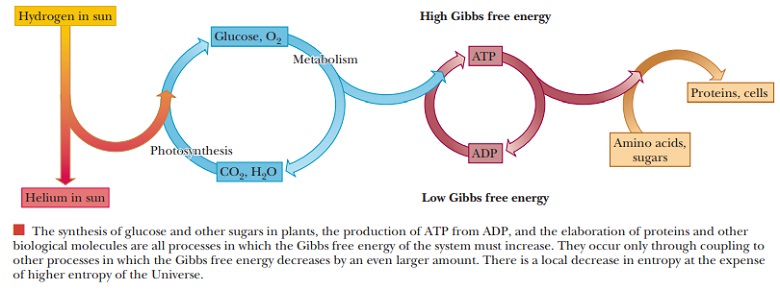
the reaction shifts to the right; if one removes reactants or adds products, the reaction shifts to the left. This is a key aspect of a number of metabolic pathways; many reactions in the middle of the pathway are likely to be freely reversible. This means that the same enzymes can be used whether the pathway is in the process of breaking down a substance or of forming the substance.
In reversible metabolic pathways, often just the reactions at the ends are irreversible, and these reactions can be turned on or off to turn the whole pathway on or off, or even to reverse it.
Driving Endergonic Reactions
Reactions can sometimes be coupled together. This occurs when the phosphorylation of glucose is coupled to the hydrolysis of one phosphate group of ATP. Of course, there are not really two reactions going on; the enzyme merely transfers the phosphate from the ATP directly to the glucose. We can think of the phosphorylation of glucose and the hydrolysis of ATP as two parts of the same reaction. We can then add them together to determine the overall energy change and make sure that, overall, it is exergonic.
Related Topics