Chapter: Biochemistry: The Importance of Energy Changes and Electron Transfer in Metabolism
Coenzymes in Biologically Important Oxidation-Reduction Reactions
Coenzymes in Biologically
Important Oxidation–Reduction Reactions
What are the reactions of key oxidation–reduction coenzymes?
The
description of redox reactions in terms of oxidation numbers, which is widely
used with inorganic compounds, can be used to deal with the oxidation of
carbon-containing molecules. However, our discussion will be more pictorial and
easier to follow if we write equations for the half reactions and then
concentrate on the functional groups of the reactants and products and on the
number of electrons transferred. An example is the oxidation half reaction for
the conversion of ethanol to acetaldehyde.
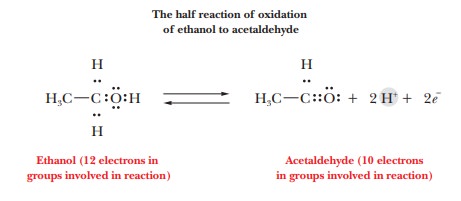
Writing
the Lewis electron-dot structures for the functional groups involved in the
reaction helps us keep track of the electrons being transferred. In the
oxidation of ethanol, there are 12 electrons in the part of the ethanol
molecule involved in the reaction and 10 electrons in the corresponding part of
the acetaldehyde molecule; two electrons are transferred to an electron
acceptor (an oxidizing agent). This type of “bookkeeping” is useful for dealing
with biochemical reactions. Many biological oxidation reactions, like this
example, are accompanied by the transfer of a proton (H+). The
oxidation half reaction has been written as a reversible reaction because the
occurrence of oxidation or reduction depends on the other reagents present.
Another
example of an oxidation half reaction is that for the conversion of NADH, the
reduced form of nicotinamide adenine dinucleotide, to the oxidized form, NAD+.
This substance is an important coenzyme
in many reactions.
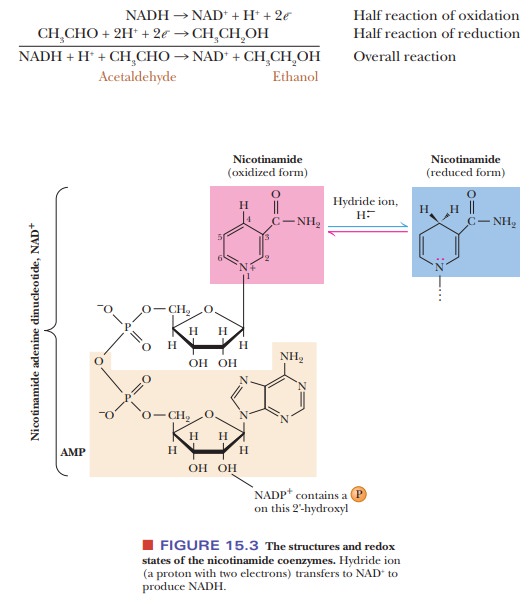
Figure
15.3 shows the structure of NAD+ and NADH; the nicotinamide
por-tion, the functional group involved in the reaction, is indicated in red
and blue. Nicotinamide is a derivative of nicotinic acid (also called niacin),
one of the B-complex vitamins. A similar compound is NADPH (for which the
oxidized form is NADP+). It differs from NADH by having an
addi-tional phosphate group; the site of attachment of this phosphate group to
ribose is also indicated in Figure 15.3. To simplify writing the equation for
the oxidation of NADH, only the nicotinamide ring is shown explicitly, with the
rest of the molecule designated as R. The two electrons that are lost when NADH
is converted to NAD+ can be considered to come from the bond between
carbon and the lost hydrogen, with the nitrogen lone-pair electrons becoming
involved in a bond. Note that the loss of a hydrogen and two electrons can be
considered as the loss of a hydride ion (H:-) by NADH and is
sometimes written that way.
The
equations for both the reaction of NADH to NAD+ and that of ethanol
to acetaldehyde have been written as oxidation half reactions. If ethanol and
NADH were mixed in a test tube, no reaction could take place because there
would be no electron acceptor. If, however, NADH were mixed with acetal-dehyde,
which is an oxidized species, a transfer of electrons could take place,
producing ethanol and NAD+. (This reaction would take place very
slowly in the absence of an enzyme to catalyze it. Here we have an excellent
example of the difference between the thermodynamic and kinetic aspects of
reactions. The reaction is spontaneous in the thermodynamic sense but very slow
in the kinetic sense.)
Such a
reaction does take place in some organisms as the last step of alcoholic
fermentation. The NADH is oxidized while the acetaldehyde is reduced.
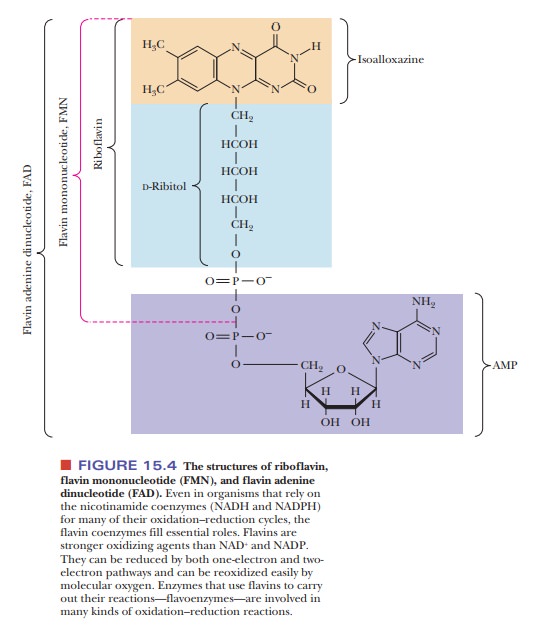
Another
important electron acceptor is FAD (flavin adenine dinucleotide) (Figure 15.4),
which is the oxidized form of FADH2. The symbol FADH2
explic-itly recognizes that protons (hydrogen ions) as well as electrons are
accepted by FAD. The structures shown in this equation again point out the
electrons that are transferred in the reaction. Several other coenzymes contain
the flavin group; they are derived from the vitamin riboflavin (vitamin B2).

Oxidation
of nutrients to provide energy for an organism cannot take place without
reduction of some electron acceptor. The ultimate electron acceptor in aerobic
oxidation is oxygen; we shall encounter intermediate electron accep-tors as we
discuss metabolic processes. Reduction of metabolites plays a signifi-cant role
in living organisms in anabolic processes. Important biomolecules are
synthesized in organisms by many reactions in which a metabolite is reduced
while the reduced form of a coenzyme is oxidized.
Summary
Two coenzymes, NADH and FADH2, play a
crucial role in biological oxidation-reduction reactions. Hydrogen ions are transferred
in addition to electrons.
Related Topics