Chapter: Biochemistry: The Importance of Energy Changes and Electron Transfer in Metabolism
The Role of Oxidation and Reduction in Metabolism
The Role of Oxidation and
Reduction in Metabolism
How are oxidation and reduction involved in metabolism?
Oxidation–reduction reactions, also referred to asredoxreactions, are those inwhich electrons are transferred from a
donor to an acceptor. Oxidation is
the loss of electrons, and reduction
is the gain of electrons. The substance that loses electrons (the electron
donor)Ñthat is, the one that is oxidizedÑis called the reducing agent or reductant. The substance that gains electrons
(the electronacceptor)Ñthe one that is reducedÑis called the oxidizing agent or oxidant. Both an
oxidizing agent and a reducing agent are necessary for the transfer of
electrons (an oxidation-reduction reaction) to take place.
An
example of an oxidation-reduction reaction is the one that occurs when a strip
of metallic zinc is placed in an aqueous solution containing copper ions. Although
both zinc and copper ions play roles in life processes, this particular
reaction does not occur in living organisms. However, it is a good place to
start our discussion of electron transfer because, in this comparatively simple
reac-tion, it is fairly easy to follow where the electrons are going. (It is
not always quite as easy to keep track of the details in biological redox
reactions.) The experimental observation is that the zinc metal disappears and
zinc ions go into solution, while copper ions are removed from the solution and
copper metal is deposited. The equation for this reaction is
Zn(s) +
Cu2+(aq) - > Zn2+(aq) + Cu(s)
The
notation (s) signiÞes a solid and (aq) signiÞes a solute in aqueous solution.
In the
reaction between zinc metal and copper ion, the Zn lost two electrons to become
the Zn2+ ion and was oxidized. A separate equation can be written
for this part of the overall reaction, and it is called the half reaction of oxida-tion:
Zn -
> Zn2+ + 2e-
Zn is
the reducing agent (it loses electrons; it is an electron donor; it is
oxidized).
Likewise,
the Cu2+ ion gained two electrons to form Cu and was reduced. An
equation can also be written for this part of the overall reaction and is
called the half reaction of reduction.
Cu2+
+ 2e- - > Cu
Cu2+
is the oxidizing agent (it gains electrons; it is an electron acceptor; it is
reduced).
If the
two equations for the half reactions are combined, the result is an equation
for the overall reaction:
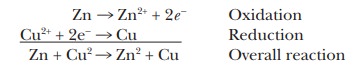
This
reaction is a particularly clear example of electron transfer. It will be
useful to keep these basic principles in mind when we examine the ßow of
electrons in the more complex redox reactions of aerobic metabolism. In many of
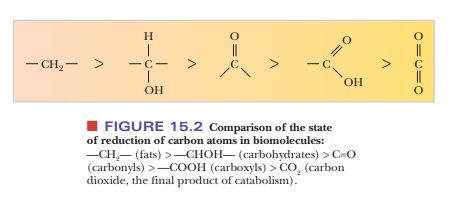
the biological redox reactions we will encounter, the oxidation state of a
carbon atom changes. Figure 15.2 shows the changes that occur as carbon in its
most reduced form (an alkane) becomes oxidized to an alcohol, an aldehyde, a
carboxylic acid, and ultimately carbon dioxide. Each of these oxidations
requires the loss of two electrons.
Summary
In catabolism, large molecules are broken down to smaller products,
releasing energy and transferring electrons to acceptor molecules of vari-ous
sorts. The overall process is one of oxidation.
In anabolism, small molecules react to give rise to larger ones;
this process requires energy and involves acceptance of electrons from a
variety of donors. The overall process is one of reduction.
Related Topics