Chapter: Biochemistry: The Importance of Energy Changes and Electron Transfer in Metabolism
Coupling of Production and Use of Energy
Coupling of Production and Use of
Energy
Another important question about metabolism is: ÒHow is the energy
released by the oxidation of nutrients trapped and used?Ó This energy cannot be
used directly; it must be shunted into an easily accessible form of chemical
energy.
We saw that several phosphorus-containing compounds, such as ATP,
can be hydrolyzed easily, and that the reaction releases energy. Formation of
ATP is intimately linked with the release of energy from oxidation of
nutrients. The coupling of energy-producing reactions and energy-requiring
reactions is a central feature in the metabolism of all organisms.
How do energy-producing reactions allow energy-requiring reactions to take place?
The
phosphorylation of ADP (adenosine diphosphate) to produce ATP (adenosine
triphosphate) requires energy, which can be supplied by the oxidation of
nutrients. Conversely, the hydrolysis of ATP to ADP releases energy (Figure
15.5).
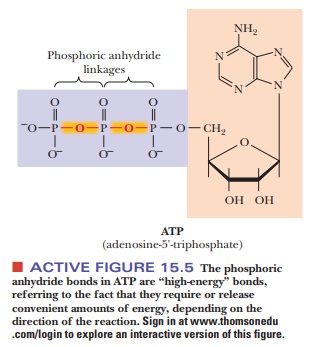
The
forms of ADP and ATP shown are in their ionization states for pH 7. The symbol
Pi for phosphate ion comes from its name in biochemi-cal jargon,
Òinorganic phosphate.Ó Note that there are four negative charges on ATP and three
on ADP; electrostatic repulsion makes ATP less stable than ADP. Energy must be
expended to put an additional negatively charged phos-phate group on ADP by
forming a covalent bond to the phosphate group being added. In addition, there
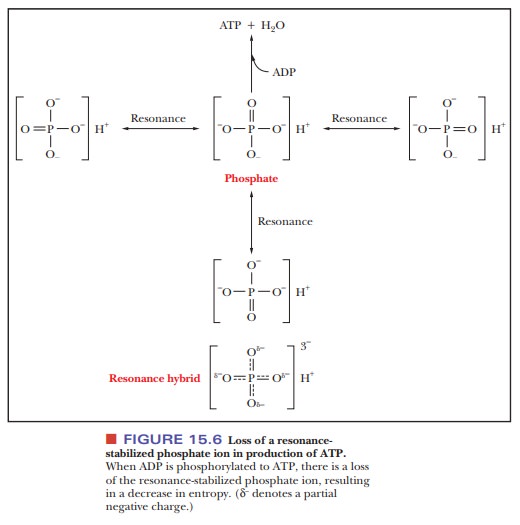
is an entropy loss when ADP is phosphorylated to ATP. Inorganic phosphate can
adopt multiple resonance structures, and the loss of these potential structures
results in a decrease in entropy when the phos-phate is attached to ADP (Figure
15.6). The ∆G° for the reaction refers
to the usual biochemical convention of pH 7 as the standard state for hydrogen
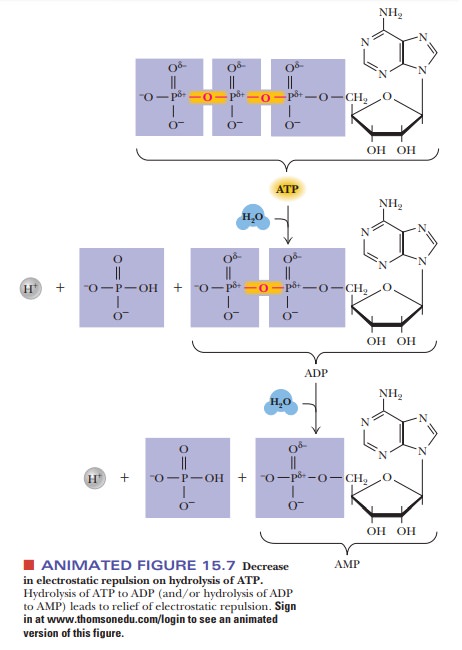
ion. Note, however, that there is a marked decrease in electrostatic repulsion
on phosphorylation of ADP to ATP (Figure 15.7).
The
reverse reaction, the hydrolysis of ATP to ADP and phosphate ion, releases 30.5
kJ mol-1 (7.3 kcal mol-1) when energy is needed:
ATP + H2O
- > ADP + Pi + H+
∆G° = -30.5 kJ mol-1 = -7.3
kcal mol-1
The bond
that is hydrolyzed when this reaction takes place is sometimes called a
Òhigh-energy bond,Ó which is shorthand terminology for a reaction in which
hydrolysis of a speciÞc bond releases a useful amount of energy. Another way of
indicating such a bond is ~P. Numerous organophosphate compounds with
high-energy bonds play roles in metabolism, but ATP is by far the most
important (Table 15.1). In some cases, the free energy of hydrolysis of
organophosphates is higher than that of ATP and is thus able to drive the
phosphorylation of ADP to ATP.
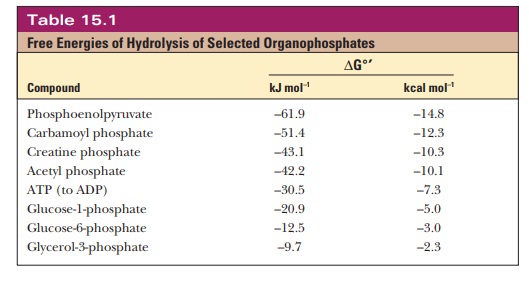
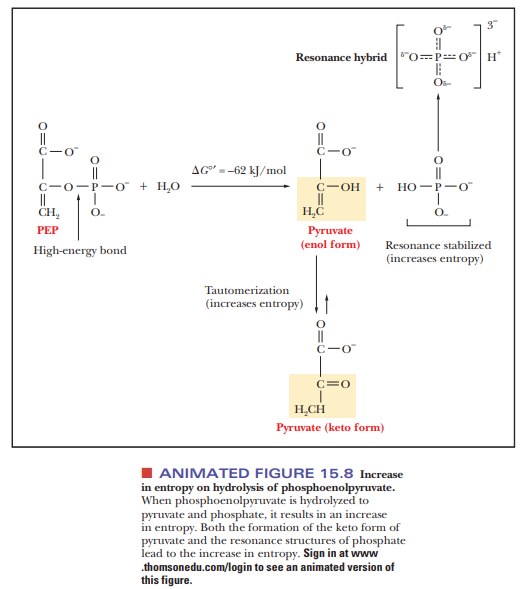
Phosphoenolpyruvate
(PEP), a molecule we shall encounter when we look at glycolysis, tops the list.
It is a very high-energy compound because of the resonance stabilization of the
liberated phosphate when it is hydrolyzed (the same effect as that seen with
ATP) and because keto-enol tautomerization of pyruvate is a possibility. Both
effects increase the entropy upon hydrolysis (Figure 15.8).
The
energy of hydrolysis of ATP is not stored energy, just as an electric cur-rent
does not represent stored energy. Both ATP and electric current must be
produced when they are neededÑby organisms or by a power plant, as the case may
be. The cycling of ATP and ADP in metabolic processes is a way of shunting
energy from its production (by oxidation of nutrients) to its uses (in
processes such as biosynthesis of essential compounds or muscle contraction)
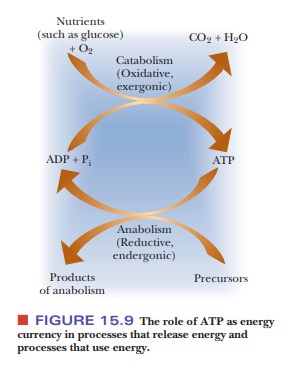
when it is needed. The oxidation processes take place when the organism needs
the energy that can be generated by the hydrolysis of ATP. When chemical energy
is stored, it is usually in the form of fats and carbohydrates, which are
metabo-lized as needed. Certain small biomolecules, such as creatine phosphate,
can also serve as vehicles for storing chemical energy. The energy that must be
sup-plied for the many endergonic reactions in life processes comes directly
from the hydrolysis of ATP and indirectly from the oxidation of nutrients. The
latter produces the energy needed to phosphorylate ADP to ATP (Figure 15.9).
Let us
examine some biological reactions that release energy and see how some of that
energy is used to phosphorylate ADP to ATP. The multistep conversion of glucose
to lactate ions is an exergonic and anaerobic process. Two molecules of ADP are
phosphorylated to ATP for each molecule of glu-cose metabolized. The basic
reactions are the production of lactate, which is exergonic,
Glucose - > 2 Lactate ions ∆G° = -184.5 kJ mol−1= -44.1 kcal mol−1
and the
phosphorylation of two moles of ADP for each mole of glucose, which is
endergonic.
2ADP +
2Pi - > 2ATP
∆G°' = 61.0 kJ mol−1= 14.6 kcal mol−1
(In the
interest of simplicity, we shall write the equation for phosphorylation of ADP
in terms of ADP, Pi, and ATP only.) The overall reaction is
Glucose
+ 2ADP + 2Pi - > 2 Lactate ions + 2ATP
∆G°' overall = -184.5 + 61.0 = -123.5 kJ mol−1= -29.5 kcal mol−1
Not only
can we add the two chemical reactions to obtain an equation for the overall
reaction, we can also add the free-energy changes for the two reactions to Þnd
the overall free-energy change. We can do this because the free-energy change
is a state function; it depends only
on the initial and Þnal states of the system under consideration, not on the
path between those states. The exergonic reaction provides energy, which drives
the endergonic reaction. This phenomenon is called coupling. The percentage of the released energy that is used to
phosphorylate ADP is the efÞciency of energy use in anaerobic metabolism; it is
(61.0/184.5) 100, or about 33%. The number 61.0 comes from the number of
kilojoules required to phosphorylate 2 moles of ADP to ATP, and the number
184.5 is the number of kilojoules released when 1 mole of glucose is converted
to 2 moles of lactate.
The
breakdown of glucose goes further under aerobic conditions than under anaerobic
conditions. The end products of aerobic oxidation are 6 molecules of carbon
dioxide and 6 molecules of water for each molecule of glucose. Up to 32
molecules of ADP can be phosphorylated to ATP when 1 molecule of glucose is
broken down completely to carbon dioxide and water.
The
exergonic reaction for the complete oxidation of glucose is
Glucose
+ 6O2 - > 6CO2 + 6H2O
∆G° = -2867 kJ mol−1= -685.9 kcal mol−1
The
endergonic reaction for phosphorylation is
32ADP +
32Pi - > 32ATP
∆G° = 976 kJ = 233.5 kcal
The net
reaction is
Glucose
+ 6O2 + 32ADP + 32Pi - > 6CO2 + 6H2O
+ 32ATP
∆G° = -2867 + 976 = -1891 kJ mol−1= -452.4 kcal mol−1
Note
that, once again, we add the two reactions and their respective free-energy
changes to obtain the overall reaction and its free-energy change. The
efÞciency of aerobic oxidation of glucose is (976/2867) 100, about 34%. (We
performed this calculation in the same way that we did with the example of
anaerobic oxidation of glucose.) More ATP is produced by the coupling process
in aerobic oxidation of glucose than by the coupling process in anaerobic
oxidation. The hydrolysis of ATP produced by breakdown (aerobic or anaerobic)
of glucose can be coupled to endergonic processes, such as muscle contraction
in exercise. As any jogger or long-distance swimmer knows, aerobic metabolism
involves large quantities of energy, processed in a highly efÞcient fashion. We
have now seen two examples of coupling of exergonic and endergonic processesÑ
aerobic oxidation of glucose and anaerobic fermentation of glucoseÑinvolving
different amounts of energy.
Summary
Hydrolysis of ATP to ADP releases energy.
In the coupling of biochemical reactions, the energy released by
one reac-tion, such as ATP hydrolysis, provides energy for another.

Related Topics