Chapter: Medical Surgical Nursing: Management of Patients With Complications From Heart Disease
Cardiac Hemodynamics
Cardiac Hemodynamics
The basic function of the heart is to pump
blood. The heart’s ability to pump is measured by cardiac output (CO), the
amount of blood pumped in 1 minute. CO is determined by measuring the heart
rate (HR) and multiplying it by the stroke volume (SV), which is the amount of
blood pumped out of the ventricle with each contraction. CO usually is
calculated using the equationCO = HR × SV.
One of the factors controlling HR is the
autonomic nervous system. When SV falls, the nervous system is stimulated to
increase HR and thereby maintain adequate CO. SV depends on three factors:
preload, afterload, and contractility.
Preload is the amount of myocardial stretch
just before systole caused by the pressure created by the volume of blood
within the ventricle. Like a rubber band, the ventricular muscle fibers need to
be stretched (by the blood) to produce optimal ejection of blood. Too little or
too much muscle fiber stretch decreases the volume of blood ejected. The major
factor that determines preload is venous return, the volume of blood that
enters the ventricle during diastole. Another factor that determines preload is
ventricular compliance, which is the elasticity or amount of “give” when blood
enters the ventricle. Elasticity is decreased when themuscle thickens, as in
hypertrophic cardiomyopathy or when there is increased fibrotic tissue within
the ventricle.Fibrotic tissue replaces dead cells, such as after a myocardial
infarction . Fibrotic tissue has little compliance,making the ventricle stiff.
Given the same volume of blood, a noncompliant ventricle has a higher
intraventricular pressure than a compliant one. The higher pressure increases
the workload of the heart and can lead to heart failure (HF).
Afterload refers to the amount of resistance
to the ejection of blood from the ventricle. To eject blood, the ventricle must
overcome this resistance. Afterload is inversely related to SV. The major
factors that determine afterload are the diameter and distensibility of the
great vessels (aorta and pulmonary artery) and the opening and competence of
the semilunar valves (pulmonic and aortic valves). The more open the valves,
the lower the resistance. If the patient has significant vasoconstriction,
hypertension, or a narrowed opening from a stenotic valve, resistance
(afterload) increases. When afterload increases, the workload of the heart must
increase to overcome the resistance and eject blood.
Contractility, which refers to the force of
contraction, is related to the
number and status
of myocardial cells.
Catecholamines, released by sympathetic stimulation such as exercise or
from administration of positive inotropic medications, can increase
contractility and stroke volume. MI causes necrosis of some myocardial cells,
shifting the workload to the remaining cells. Significant loss of myocardial
cells can decrease contractility and cause HF. Afterload must be reduced by
stress reduction techniques or medications to match the lower contractility.
NONINVASIVE ASSESSMENT OF CARDIAC HEMODYNAMICS
Several
noninvasive assessment findings can indicate cardiac he-modynamic status,
although the findings do not directly correlate to preload, afterload, or
contractility. Right ventricular preload may be estimated by measuring jugular
venous distention. Elevated left ventricular preload may be identified by a
positive hepatojugu-lar test. Mean arterial blood pressure is a rough indicator
of left ven-tricular afterload. Activity tolerance may be used as an indicator
of overall cardiac functioning.
Impedance
cardiography (ICG) is a noninvasive method for continuous calculation of SV,
CO, systemic vascular resistance, ventricular contractility, and fluid status
(Turner, 2000). Elec-trodes are placed on the patient’s chest. The electrodes
are con-nected to a device that transmits a very small amount of alternating
electric current through the chest and measures the resistance (Z) to the flow
(conduction) of the current. Because the current seeks the path of least
resistance and fluid is an excellent conductor, the current flows through the
blood. ICG measures the volume of blood flow.
The
cardiac cycle produces normal changes in blood flow vol-ume; for example, there
is more blood flow volume during systole and less blood flow volume during
diastole. The changes in blood flow volume change the resistance to flow of the
current, which is called electrical impedance (dZ). During systole, the higher
blood flow volume causes the red blood cells to be aligned in a more par-allel
pattern, which makes the flow of current faster and reduces impedance. During
diastole, the lower blood flow volume causes the red blood cells to be more
randomly arranged, which makes the flow of current slower and increases
impedance. Stroke vol-ume is determined by comparing dZ to the changes in time
(dt) (Von Rueden & Turner, 1999). The preejection period (PEP) and
ventricular ejection times (VET) can be measured, which further assists in
understanding the hemodynamic status of the patient. For example, a
dysfunctional left ventricle requires more time to generate pressure to
overcome the resistance to ejection so that the aortic valve opens (increased
PEP) and has less time during which blood is ejected into the aorta (decreased
VET).
INVASIVE ASSESSMENT OF CARDIAC HEMODYNAMICS
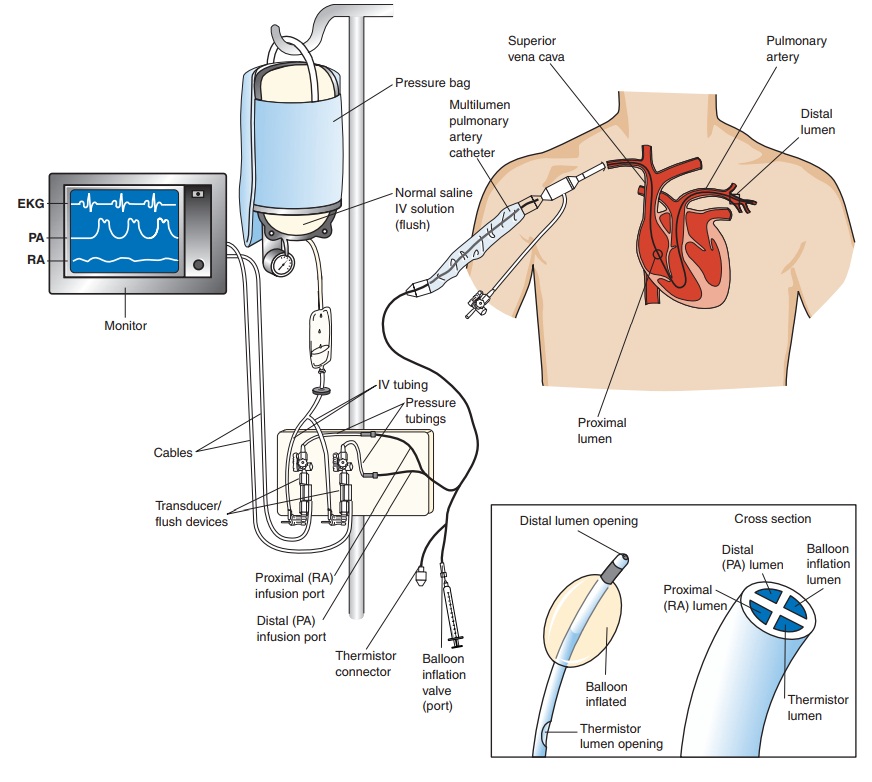
An important
method for evaluating the components of SV in a hemodynamically unstable
patient is the pulmonary artery (PA) catheter, which is used to obtain the
hemodynamic data essential for diagnosis and treatment . Connected to a
com-puterized transducer apparatus, the PA catheter serves as a fluid-filled
conduit for detecting pressure changes within the heart. The pulsatile changes
in pressure are converted into electrical sig-nals, which are displayed as
waveforms on a monitor (Fig. 30-1; Chart 30-1).
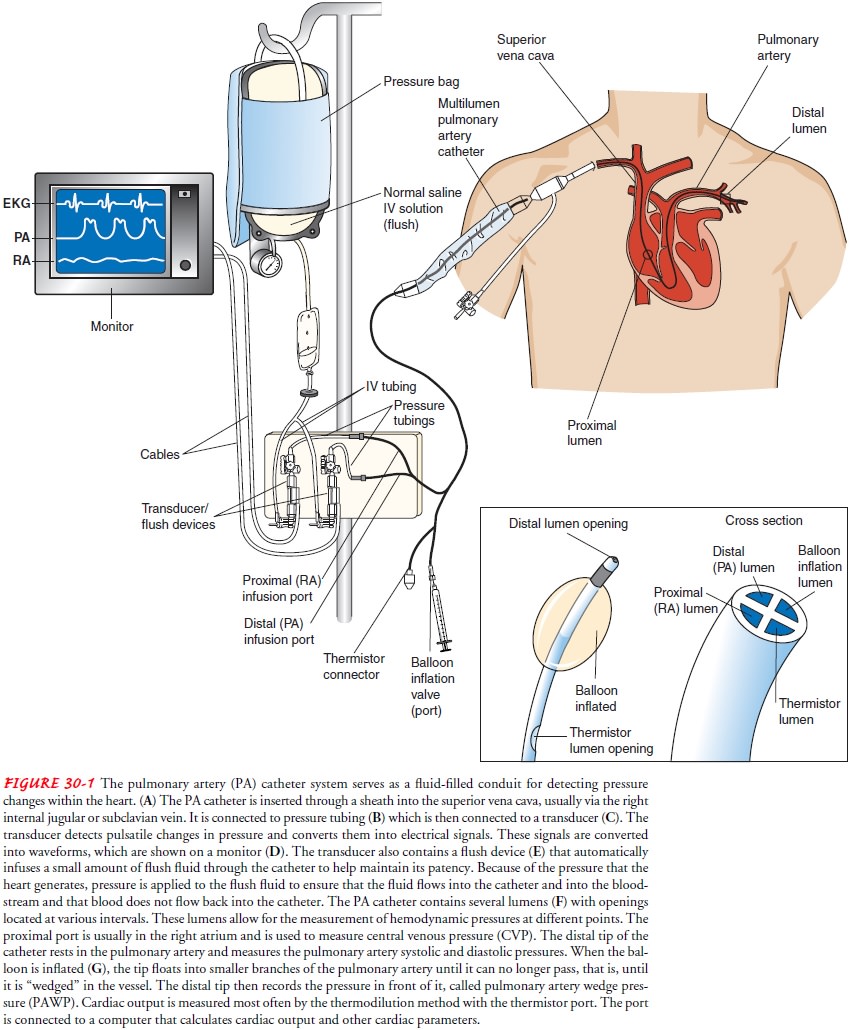


CO is
measured most often by the thermodilution
method with the thermistor port of the catheter. The port is connected to a
computer that calculates CO and other cardiac parameters. In thermodilution, a
specific volume of fluid that is colder than the patient’s blood is injected
into the proximal port (right atrium). The fluid enters the right ventricle and
is then ejected into the PA. The thermistor records the temperature before and
after the ejection of fluid. The change in temperature is inversely related to
CO; the greater the CO, the faster the blood and fluid moves, the less time the
fluid has to mix with the blood to cause a changein temperature, and the less
change in temperature detected by the thermistor.
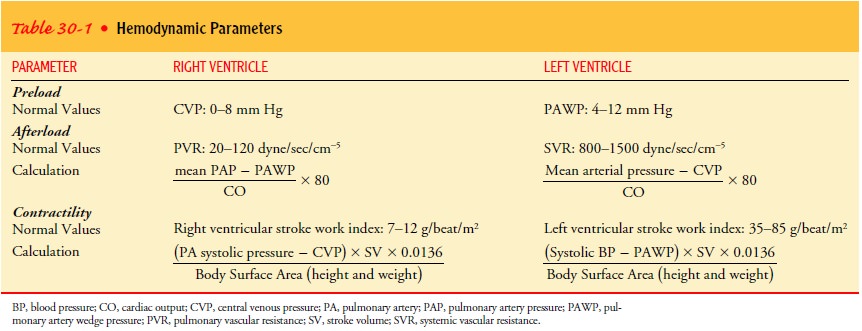
Cardiac
parameters for afterload and contractility are calcu-lated at the same time as
CO (Table 30-1). Measurements of the various pressures are made at intervals.
Therapy, especially intra-venous medication, is adjusted based on the
assessment and di-agnostic findings.
The
patient with an invasive hemodynamic catheter is usu-ally managed in an
intensive care environment (see Chart 30-1) because of the need for frequent
nursing assessments and inter-ventions.
Related Topics