Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
Acids, Bases, and pH
Acids, Bases, and pH
The
biochemical behavior of many important compounds depends on their acid–base
properties.
What are acids and bases?
A
biologically useful definition of an acid is a molecule that acts as a proton
(hydrogen ion) donor. A base is similarly defined as a proton acceptor. How
readily acids or bases lose or gain protons depends on the chemical nature of
the compounds under consideration. The degree of dissociation of acids in
water, for example, ranges from essentially complete dissociation for a strong
acid to practi-cally no dissociation for a very weak acid, and any intermediate
value is possible.
It is
useful to derive a numerical measure of acid
strength, which is the amount of hydrogen ion released when a given amount
of acid is dissolved in water. Such an expression, called the acid dissociation constant, or Ka, can be
written for any acid, HA, that reacts according to the equation
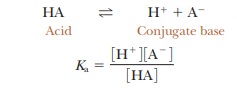
In this
expression, the square brackets refer to molar concentration-that is, the
concentration in moles per liter. For each acid, the quantity Ka has a
fixed numerical value at a given temperature. This value is larger for more
completely dissociated acids; thus the greater the Ka, the stronger the acid.
Strictly
speaking, the preceding acid–base reaction is a proton-transfer reac-tion in
which water acts as a base as well as the solvent. A more correct way to write
the equation is as follows:
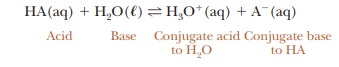
The
notation (aq) refers to solutes in aqueous solution, whereas ( ) refers to
water in the liquid state. It is well established that there are no “naked
protons” (free hydrogen ions) in solution; even the hydronium ion (H3O1) is an
underestimate of the degree of hydration of hydrogen ion in aqueous solution.
All solutes are extensively hydrated in aqueous solution. We will write the
short form of equations for acid dissociation in the interest of simplicity,
but the role of water should be kept in mind throughout our discussion.
What is pH?
The
acid–base properties of water play an important part in biological processes
because of the central role of water as a solvent. The extent of
self-dissociation of water to hydrogen ion and hydroxide ion,
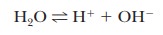
is
small, but the fact that it takes place determines important properties of many
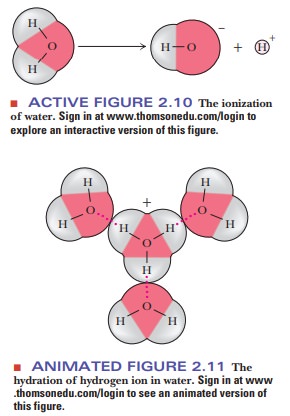
solutes (Figure 2.10). Both the hydrogen ion (H+) and
the hydroxide ion (OH-) are associated with several
water molecules, as are all ions in aqueous solution, and the water molecule in
the equation is itself part of a cluster of such molecules (Figure 2.11). It is
especially important to have a quantitative estimate of the degree of
dissociation of water. We can start with the expression
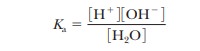
The
molar concentration of pure water, [H2O], is
quite large compared to any possible concentrations of solutes and can be
considered a constant. (The numerical value is 55.5 M, which can be obtained by dividing the number of grams of water
in 1 L, 1000 g, by the molecular weight of water, 18 g/mol; 1000/18 5 55.5 M.) Thus,
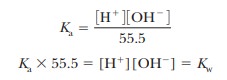
A new
constant, Kw, the ion product constant for water, has
just been defined, where the concentration of water has been included in its
value.
The
numerical value of Kw can be
determined experimentally by measuring the hydrogen ion concentration of pure
water. The hydrogen ion concentra-tion is also equal, by definition, to the
hydroxide ion concentration because water is a monoprotic acid (one that
releases a single proton per molecule). At 25°C in pure water,
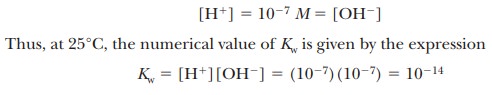
This
relationship, which we have derived for pure water, is valid for any aqueous solution, whether neutral,
acidic, or basic.
The wide
range of possible hydrogen ion and hydroxide ion concentrations in aqueous
solution makes it desirable to define a quantity for expressing these
concentrations more conveniently than by exponential notation. This quantity is
called pH and is defined as
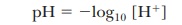
with the
logarithm taken to the base 10. Note that, because of the logarithms involved,
a difference of one pH unit implies a tenfold difference in hydrogen ion
concentration, [H+]. The pH values of some
typical aqueous samples can be determined by a simple calculation.

When a
solution has a pH of 7, it is said to be neutral,
like pure water. Acidic solutions have pH values lower than 7, and basic
solutions have pH values higher than 7.
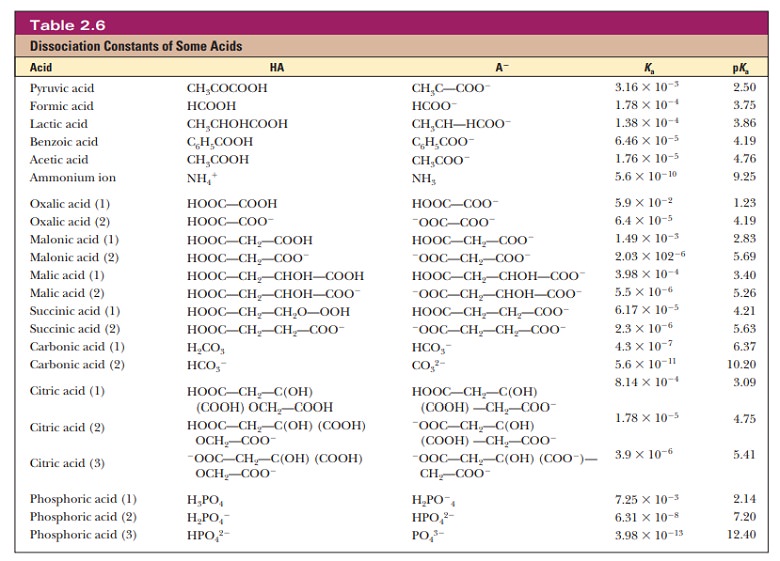
In
biochemistry, most of the acids encountered are weak acids. These have a Kawell
below 1. To avoid having to use numbers with large, negative exponents,a
similar quantity, pKa, has
been defined by analogy with the definition of pH:
pKa= -log10Ka
The pKa is a
more convenient numerical measure of acid strength. The smaller its value, the
stronger the acid. This is the reverse of the situation with Ka, where
larger values imply stronger acids (Table 2.6).
Why do we want to know the pH?
An
equation connects the Ka of any
weak acid with the pH of a solution containing both that acid and its conjugate
base. This relationship has wide use in biochemistry, especially where it is
necessary to control pH for optimum reaction conditions. Some reactions cannot
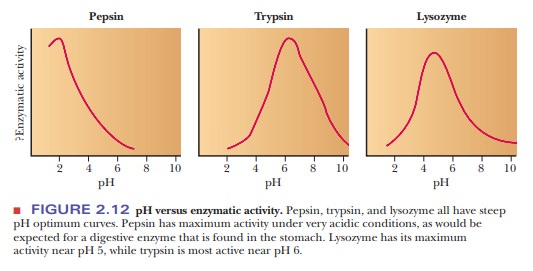
take place if the pH varies from the optimum value. Important biological
macromolecules lose activity at extremes of pH. Figure 2.12 shows how the
activities of three enzymes are affected by pH. Note that each one has a peak
activity that falls off rapidly as the pH is changed from the optimum. Also,
some drastic physiological consequences can result from pH fluctuations in the
body. To derive the involved equation, it is first necessary to take the
logarithm of both sides of the Ka
equation.
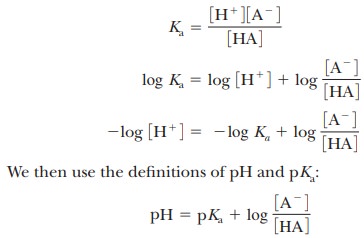
This relationship is known as the Henderson–Hasselbalch equation and is useful in predicting the properties of buffer solutions used to control the pH of reaction mixtures.
When buffers are discussed, we will be interested in the
situation in which the concentration of acid, [HA], and the concentration of
the conjugate base, [A-], are equal ([HA] = [A-]). The
ratio [A-]/[HA] is then equal to 1, and the logarithm of 1 is equal to zero.
Therefore, when a solution contains equal concentrations of a weak acid and its
conjugate base, the pH of that solution equals the pKa value of the weak acid.
Summary
Acids are proton donors, and bases are proton
acceptors.
Water can accept or donate protons.
The strength of an acid is measured by its acid
dissociation constant, Ka. The
larger the Ka, the
stronger the acid and the more H+ dissociates.
The concentration of H+ is
expressed conveniently as the pH, which is the negative log of the hydrogen ion
concentration.
A similar expression, pKa, can be used in place of the Ka. pKa5 2log Ka.
The pH
of a solution of a weak acid and its conjugate base is related to the
concentration of the acid and base and the pKa by the
Henderson–Hasselbalch equation.
Related Topics