Chapter: Civil : Remote Sensing Techniques and GIS : EMR and Its Interaction With Atmosphere and Earth Material
Wave Theory and Parrtical Theory
WAVE THEORY AND PARRTICAL THEORY
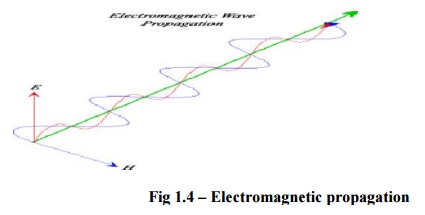
Light can
exhibit both a wave theory, and a particle theory at the same time. Much of the
time, light behaves like a wave. Light waves are also called electromagnetic
waves because they are made up of both electric (E) and magnetic (H)
fields. Electromagnetic fields oscillate perpendicular to the direction of wave
travel, and perpendicular to each other. Light waves are known as transverse
waves as they oscillate in the direction traverse to the direction of wave
travel.
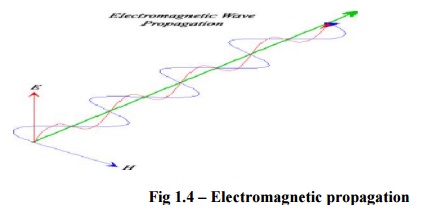
Fig 1.4 -
Electromagnetic propagation
Waves have two important characteristics - wavelength and
frequency.
The sine wave is the fundamental
waveform in nature. When dealing with light waves, we refer to the sine wave.
The period (T) of the waveform is one full 0 to 360 degree sweep. The
relationship of frequency and the period is given by the equation:
f = 1 / T
T = 1 / f
The waveforms are always in the time domain and go on for
infinity.
The speed of a wave can be found
by multiplying the two units together. The wave's speed is measured in units of
length (distance) per second:
Wavelength x Frequency = Speed
As
proposed by Einstein, light is composed of photons, a very small packets of
energy. The reason that photons are able to travel at light speeds is due to
the fact that they have no mass and therefore, Einstein's infamous equation - E=MC2
cannot be used. Another formula devised by Planck, is used to describe the
relation between photon energy and frequency
Planck's Constant
(h) - 6.63x10-34 Joule-Second.
E = hf(or)E
= hc / /?
E is the
photonic energy in Joules, h is Planks constant and f is the frequency in Hz.
PARTICAL THEORY
The basic idea of qua ntum theory
is that radiant energy is trans mitted inindivisible packets whose energy is
given in integral parts, of size hv, where h is Planck's constant = 6.6252 x
10-34 J - s, and v is the frequency of the radiation. These ar e called quanta
or photons.
The dilemma of the si multaneous
wave and particle waves of elec tromagneticenergy may be conceptually resolved
by considering that energy is not supplied continuously throughout a wave, but
rather that it is carried by photons. The classical w ave theory does not give
the intensity of energy at a point in space, but gives the probability of
finding a photon at that point. Thus the classica l concept of a wave yields to
the idea th at a wave simply describes the probability path for the motion of
the individual photons.
The particular impor tance of the
quantum approach for remote sensing is thatit provides the concept of discrete
energy levels in materials. The values a nd arrangement of these levels are
different for different materials. Information about a given material is thus
available in electromagnetic radiation as a consequence of transitions bettween
these energy levels. A transition to a highe r energy level is caused by the
absorption of energy, or from a higher to a lower energy leve l is caused by
the' emission of energy. The amounts of energy either absorbed or emitted c
orrespond precisely to the energy difference between the two levels involved in
the transitio n. Because the energy levels are different for each material, the
amount of energy a particular substance can absorb or emit is different for
that material from any other materials. Conseque ntly, the position and
intensities of the band s in the spectrum of a given material are
characteriistic to that material.
Related Topics