Chapter: Pharmaceutical Drug Analysis: Theory and Technique of Quantitative Analysis
Volumetric Analysis
VOLUMETRIC ANALYSIS
Volumetric analysis may be broadly defined as those
analytical methods whereby the exact volume of a solution of known
concentration actually consumed during the course of an analysis is considered
as a measure of the amount of active constituent in a given sample under
determination (assay).
1. THEORY
According to the official method of analysis,
hydrochloric acid can be determined by first
weighing a given sample accurately, and secondly,
by adding carefully a solution of known strength of sodium hydroxide in the
presence of an appropriate indicator unless and until the exact equivalent
amounts of HCl and NaOH have undergone the following chemical reaction :
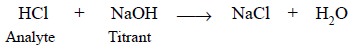
Analyte (or Active
Constituent) is
the chemical entity under assay e.g., HCl.
Titrant is the solution of known
strength (or concentration) employed in the assay e.g., NaOH.
Titration is the process of adding and
then actually measuring the volume of titrant consumed in the assay. This volume is usually measured
by the help of a calibrated burette.
Indicator is a chemical substance sensitive
enough to display an apparent change in colour very close to the point in the ongoing titration
process at which equivalent quantities of analyte and titrant have almost
virtually reacted with each other.
Equivalence Point (or
Stoichiometric Point) is the point at which there appears an abrupt change in certain characteristic of the
prevailing reaction mixture—a change that is either ascertained
electrometrically or is visibly spotted by the use of indicators.
In usual practice, the volumetric titrations may be
accomplished either by direct titration method e.g., assay of HCl employing NaOH as the titrant, or by residual
titration method e.g., assay of ZnO
in which case a known-excess-measured volume of standardised solution of H2SO4,
more than the actual amount chemically equivalent to ZnO, is added to the
sample ; thereupon, the H2SO4 which remain unreacted with
ZnO is subsequently titrated (sometimes referred to as back titration or residual
titration in the text) employing standardized NaOH solution.
Thus, we have :
Known amount of H2SO4 consumed ≡
Known amount of NaOH + Unknown amount of ZnO Most official compendia usually record the results of drug assays in
terms of % w/v, % w/w and % v/v.
2. DEFINITIONS
In order to have a clear-cut understanding of the various
calculations involving volumetric assays through-out this book one needs to
gain an in-depth knowledge of the various terms related to ‘equivalents’. They are :
(a) Gram-equivalent Weight (GEW) : It is
the weight in grams that is chemically equivalent to 1 gram-atom of hydrogen
(1.0079 g).
It is also sometimes simply referred to as the
‘gram-equivalent’. However, GEW has two distinct definitions for neutralization
as well as as oxidation-reduction reactions as stated below :
(i)
For Neutralization Reactions : GEW is defined as that weight of a substance in grams which
contains, furnishes, reacts directly or
indirectly and replaces 1 gram-atom or ion of hydrogen.
(ii) For Oxidation—Reduction
Reactions
Explanation : A reaction usually takes place
by the combination of oxidizing and reducing agents and this may be considered as the basis for the quantitative
measurement of one of the reactants. For instance, FeSO4 can be
determined quantitatively by its reaction with ceric sulphate [Ce(SO4)2]
as expressed by the following equation :
 .......(a)
.......(a)
Equation (a)
can be split into two half-equations as shown below thereby depicting the loss
of elec-trons by the Fe2+ ion [Eq. (b)] and the gain of electrons by the Ce4+ [Eq. (c)] :
 ...........(b)
...........(b)
 .................(c)
.................(c)
From Eq. (a) it
is evident that each molecule of FeSO4, upon oxidation, happens to
lose one electron. Hence, one mole of FeSO4 loses 6.02 × 10 23
electrons which is equivalent to 1 Faraday or 96,500 C. Thus, in
electrochemical determination of equivalence point the quantity of electricity
is almost identical with that required to reduce 1 mole of Ce(SO4)2.
It follows from here that 1 mole of FeSO4 and 1 mole of Ce(SO4)2
are chemical equivalents. In other words, 1 g of H, acting as a reducing agent,
loses electrons equivalent to 96,500 C.
(b) Equivalent Weight of a Reducing Agent
is that weight which loses electrons equivalent to 96,500 C.
It may be calculated by dividing the gram-molecular
weight by the number of electrons lost by each molecule, for instance :
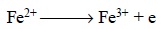
hence, the equivalent weight of FeSO4
oxidizing to Fe2(SO4)3 comes out to be 151.919
[FeSO4 :
molecular weight = 151.91] or 1 gram-molecular weight.
(c) Equivalent Weight of an Oxidizing Agent
is that weight which gains electrons equivalent to 1 Faraday, or to the
electrons gained by 1 gram-ion of H+ ions (2H+ + 2e → H2 ).
It may be calculated by dividing the gram-molecular weight by the number of electrons gained by each molecule, for example :
(a) Ce4+ + e → Ce3+ (cerous
ion)
hence, the equivalent weight of ceric sulphate is 1
gram-molecular weight 332.24 g [Ce(SO4)2 : molecular
weight = 332.24]
(b) MnO4– + 5e → Mn2+ (manganous
ion)
hence, the equivalent weight of potassium permanganate is
1/5th gram-molecular weight 31.61 g.
(KMnO4 : 1/5 × 158.05 = 31.61)
(c) Cr2O72– +
6e —→ 2Cr3+ (chromous ion)
hence, the equivalent weight of potassium dichromate is
l/6 gram-molecular weight 49.03 g. (K2Cr2O7 :
1/6 × 294.18 = 49.03)
(d) I2 + 2e → 2I– (iodide ion)
hence, the equivalent weight of iodine is 1
gram-molecular weight 126.90 g. (I2 : Molecular Weight = 126.90)
(e) BrO3–
+ 6e → Br– (bromide
ion)
hence, the equivalent weight of potassium bromate is 1/6
gram-molecular weight 27.83 g.
(KBrO3 : 1/6 × 167.01 = 27.83)
(d) Gram-milliequivalent Weight (GmEW) is
nothing but GEW/1000. This term is very much used in all types of volumetric
calculations.
(e) Equivalent (equiv) is the number of
gram-equivalents involved in a quantitative method.
(f) Milliequivalent (meq) is the number of
gram-milliequivalents involved in a quantitative method.
However, meq is used more frequently than equiv in
quantitative procedures.
(g) Standard Solution is a solution of
known (pre-determined) normality or molarity.
(h) Normality (expression of concentration)
is the number of equivalents of solute per litre (equiv/lire) or
milliequivalents per ml. (meg/ml) solution.
(i) Molarity is the expression of the
concentration of a solution in terms of moles per litre.
(j) Standardization is the actual
determination of either the normality or the molarity of a solution.
(k) Primary Standard is the substance of
known purity (‘AnalaR’-grade reagents) whose carefully weighed quantity helps
in the standardization of an unknown solution (normality or molarity).
(l) Secondary Standard is another standard
solution that is used for standardization of an unknown solution.
Example : An unknown solution of HCl may
be standardized volumetrically in two
ways, namely :
(i) by the help
of ‘AnalaR’-grade Na2CO3 i.e., purity is known-‘Primary
Standard’, and
(ii) by the
help of another standard solution of NaOH—‘Secondary
Standard’.
(m) Titer : is the weight of a substance
chemically equivalent to 1 ml of a standard solution.
Example : 1 ml of 1 N HCl contains
0.03646 g (i.e., 0.001 equiv or 1
meq) of HCl and hence is chemically
equivalent to 0.04000 g (i.e., 0.001
equiv or 1 meq) of NaOH.
Thus, most calculations in volumetric determinations
(titrimetry) are enormously facilitated by using titer values.
For instance, in the offcial procedure for the assay of
tartaric acid, it is stated that ‘Each millilitre of 1 N sodium hydroxide is
equivalent to 75.04 mg of C4H6O6’. The C4H6O6
titer of 1 N sodium hydroxide is, therefore, 75.04 mg/ml, a value that may be
calculated as follows :
An examination of the equation indicates that 1 mole or
150.09 g of 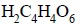
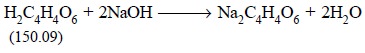
is 2 equiv, and the equivalent weight of H2C4H4O6
is 75.04 g. Hence, each millilitre of 1 NaOH contains 0.001 equiv of NaOH and
is equivalent to 0.001 equiv or 0.001 × 75.04 = 0.07504 g or 75.04 mg of H6C4O6.
Related Topics