Chapter: Pharmaceutical Drug Analysis: Theory and Technique of Quantitative Analysis
Biomedical Analytical Chemistry: Colorimetric Assays
COLORIMETRIC ASSAYS
A. Theory : In fact, two fundamental laws actually govern the practice of colorimeteric
assays of photometry.
First Law : Bougner’s (1729) or Lambert’s (1760) Law defines
that—“when a beam of monochromatic light, previously rendered plane-parallel, enters an
absorbing medium at right angles to the plane-parallel surfaces of the medium,
the rate of decrease in radiant power with the length of light path through the
absorbing medium `b’ is directly
proportional to the radiant power of the beam, i.e., the light will be
diminished in geometric (not arithmetic) or exponential progression”.
Alternatively, it may be explained that if a particular thickness
absorbs half the light, the thickness which follows the first half and is equal
to it will not absorb the entire second half, but instead only half of this
half and will consequently reduce it to one-quarter. Thus, we have :
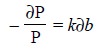
Upon integration and changing to logarithms of base 10,
and substituting P = P0 when b
= 0, we may get :
2.303 log (P0/P) = kb ... (b)
In other words, the radiant power of the unabsorbed light
decreases exponentially as the thickness of the absorbing medium increases
arithmetically,
P = P0 e–kb
= P0 10–0.43 kb...(c)
Second Law : Bernard’s (1852) or Beer’s (1852) Law defines that—‘the
radiant power of a beam of parallel monochromatic radiation decreases in a similar
manner as the concentration of the light-absorbing constituent increases”. Thus we have :
2.303 log (P0/P) = k′ C ... (d)
where, C = concentration of substance, and
k′ = constant of proportionality.
Therefore, from Eq. (b)
and Eq. (d), the two Laws may be
combined and expressed with a single constant as follows :
log (P0/P) = abc ... (e)
or P = P0 10–abc ... (f)
where, a =
absorptivity constant*.
[* and not to be
tenned as absorbancy index, extinction coeffcient or specific extinction.]
In fact, the absorptivity constant ‘a’ is dependent upon the wavelength of the radiation as well as the
nature of the absorbing material, whose concentration ‘C’ is usually expressed
in grams per litre.
Molar Absorptivity (∈) : It is the product of the molecular weight of the
substance and its absorptivity and
is designated by the symbol ∈.
Beer’s Law (or Beer-Lambert’s
Law) : The
combined law is invariably referred to as ‘Beer’s
Law’, while some texts refer to
this as ‘Beer-Lambert’s Law’.
Eq. (f) is
mostly expressed as shown below :
A = abc ...(g)
where, A = absorbance,
a = absorptivity,
b = optical path length, and
c = analyte concentration.
The term A1%1cm designates the
absorbance of a 1 cm layer of solution that essentially contains 1% by weight
of absorbing solute.
It is pertinent to mention here that most of the pure
pharmaceutical substances (RS) do possess a definite characteristic absorbance
(i.e., A1%1cn )
that forms the basis of their assay vis-a-vis
the unknown sample.
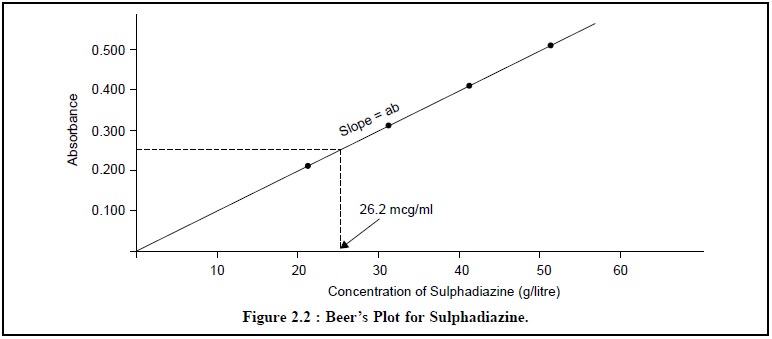
Beer’s Plot : It is a plot of the absorbance value (along Y-axis)
against a series of unknown solute concentrations in g/litre (along X-axis) thereby yielding
a straight line passing through the origin.
Therefore, the solute-concentration present in an unknown
solution can be estimated conveniently from the Beer’s Plot or sometimes referred to as the Standard Curve, merely by measuring the absorbance value of the
solution and then finding the concentration value that corresponds to the measured
absorbance value as is illustrated in the following Figure 2.2.
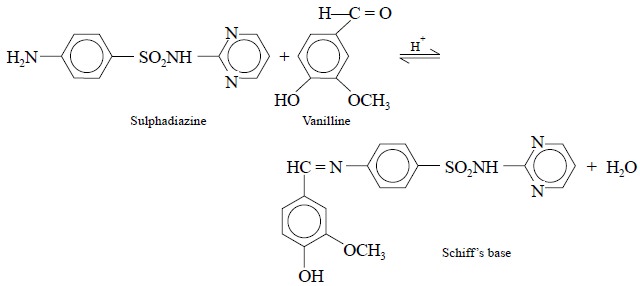
The colorimetric assay of sulphadiazine is based on the acid-catalysed equilibrium reaction
that occurs between vanillin (an aldehyde) and sulphadiazine (an arylamine).
The chemical species that forms as shown below is known as the Schiff’s Base and is yellow in colour.
Transmittance. The relationship between per
cent transmittance and concentration is shown in Figure 2.3.
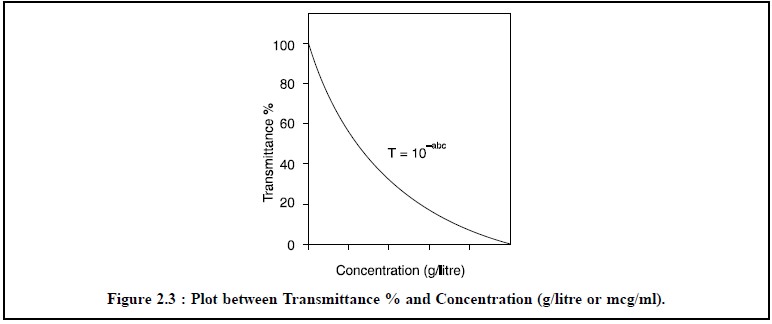
From Figure 2.3, it is quite evident that at lower
concentrations the per cent trasmission is high and is vice varsa at higher concentrations.
However, a direct relationship between per cent
transmittance and absorbance is illustrated in Figure 2.4.

B. Applications in Biomedical
Analytical Chemistry Colorimetric assays have a wide spectrum of applications in biomedical analytical
chemistry which may be categorized under the following four heads, namely :
(i)
Colorimetric Assays of Biochemicals,
(ii)
Colorimetric Assays Involving Complexation Reactions,
(iii)
Colorimetric Assays Involving Redox Reactions, and
(iv)
Colorimetric Assays of Enzyme Levels.
All these four categories of colorimetric assays shall be
discussed briefly with appropriate examples, wherever necessary, to have an
indepth knowledge and better understanding of the practical aspects.
1. Colorimetric Assays of Biochemicals
In this context, the discussion shall be restricted to
the colorimetric assays of urea (BUN), bilirubin and cholesterol. However, the
clinical significance of these substances and the extent to which they are
present in biological fluids; besides the various drugs that usually interfere
with their assay are also described adequately in the following pages :
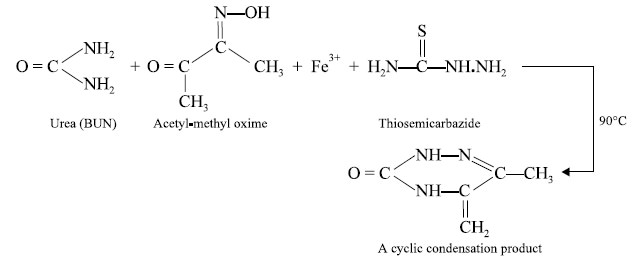
1.1. Urea (BUN)
The extent of urea (BUN) present in biological fluids is
normally determined in many Auto Analyzers by the following method :
The quantity of substance having an unknown structure is
determined at 520 nm spectrophotometrically, while the normal BUN level is
determined by averaging the BUN levels of a number of normal subjects.
Profile of BUN-levels
·
normal BUN level is 10-15 mg per 100 ml,
·
Enhanced BUN levels clearly signify a renal dysfunction,
for instance urinary tract obstruction and nephritis i.e., inflammation of the kidney.
·
Increased incidence of BUN is also found in subjects
suffering from diabetes, hepatic disorders and gastrointestinal disturbances,
·
Decreased BUN level is usually indicative of acute
hepatic dysfunction and excessive dehydration,
·
A few important drugs, namely : thiazide diuretics (e.g., chlorothiazide,
hydroflumethiazide, bendroflumethiazide, benzthiazide, cyclothiazide etc.),
neomycin, tetracyclines, methyldopa etc., help in enhancing the BUN levels
perhaps due to interference with normal renal function,
·
Phenothiazines (e.g.,
promethazine, chlorpromazine, ethopropazine etc.) on the contrary causes a
significant decrease in BUN levels due to lowering of urea production from the
liver, and
·
Substances that are inherently present in the serum and
absorb at 520 nm shall interfere with these measurements, and therefore,
necessary corrections for these materials have got to be made adequately.
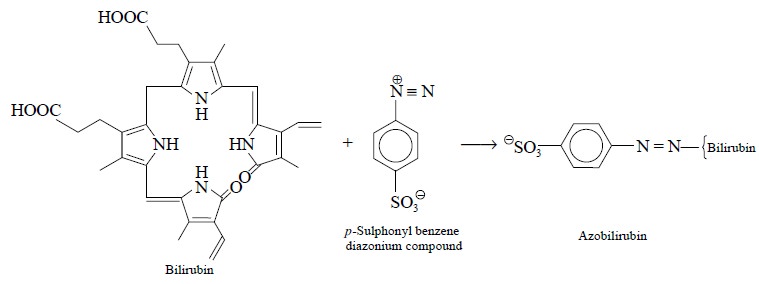
1.2. Bilirubin
Bilirubin is diazotized with para-sulphonyl benzene diazonium
compound and the absorbance of the resulting azobilirubin is measured at 600 nm
to determine bilirubin level in the biological fluid e.g., blood serum. In usual practice, a serum blank is run
simultaneously by reacting the serum with caffeine, sulphanilic acid and
tartaric acid, and the absorbance of the blank is measured at 600 nm which is
subsequently subtracted from the azobilirubin absorbance initially obtained
before the bilirubin level is finally determined.
Profile of Bilirubin Levels
·
Normal bilirubin level ranges between 0-1.5 mg per 100
ml,
·
Enhanced bilirubin level may suggest drug toxicity,
bile-tract obstruction, hepatitis and hepatic dysfunction,
·
As normal bilirubin level commences from zero, hence
conditions responsible for its decrease are practically non-existent,
·
Increased bilirubin levels are caused due to the intake
of large doses of such drugs as : chloroquine, vitamin K, sulpha-drugs,
tetracyclines, paracetamol, nicotinic acid and monoamine oxidase inhibi-tors (e.g., iproniazid RP 1.0 ; nialamide RP
1.8 ; isocarboxazid RP 3.1 ; phenelzine RP 18 ; pheniprazine RP3l ; and
tranylcypromine RP 45), where RP designates the ‘Relative Potency’ based on the
tryptamine potentiation test. The elevated levels are due to hepatic injury,
and
·
Drugs that interfere with the assay are, namely : (a) phenylazopyridine hydrochloride—a
coloured drug, (b) azo-compound
forming medicinals, and (c)
degradation product of novobiocin.
1.3. Cholesterol
Cholesterol interacts with
glacial acetic acid and acetic anhydride to result into the formation of a
coloured product whose absorption is measured at 630 nm and this is found to be
directly proportional to the level of cholesterol present in the serum. The
reaction may be expressed as follows :
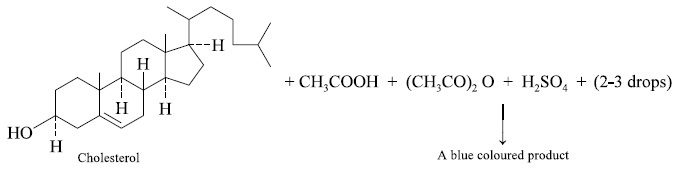
The above reactions is also referred to as the
Libermann’s Reaction.
Profile of Cholesterol Levels
·
Normal total cholesterol level is 200 mg per 100 ml,
·
Increased cholesterol levels in serum are found in
patients suffering from chronic hepatitis, atherosclerosis (deposit of fat in
arteries of heart) and hypothyroidism,
·
Decreased cholesterol levels in serum is indicative of
liver ailment and hyperthyroidism,
·
Corticosteroids (i.e.,
steroidal compounds) found in urine that possess biological properties
resembling those of adrenal cortical extract, either in the increase or
decrease of cholesterols levels,
·
Oestrogens, for instance : estrone, estriol, estradiol
etc., are found to lower the cholesterol levels,
·
The broad-spectrum antibiotic chlorotetracycline and the
aminoglycoside antibiotic kanamycin are observed to lower the cholesterol
levels by forming salts with bile acids (e.g.,
cholic acid, deoxycholic acid and chenodeoxycholic acid) in the intestinal
canal,
·
Likewise, the antoconvulsant phenytoin sodium and
neomycin—an aminoglycoside antibiotic also decrease the cholesterol levels, and
·
Interestingly, penicillamine—a degradation product of
penicillin and phenothiazines—the histamine H1—receptor antagonists,
such as : promethazine teoclate, methadilazine hydrochloride, trimeprazine
tartrate are found to increase the cholesterol levels.
Related Topics