Chapter: 11th Biochemistry : Chapter 10 : Biochemical Techniques
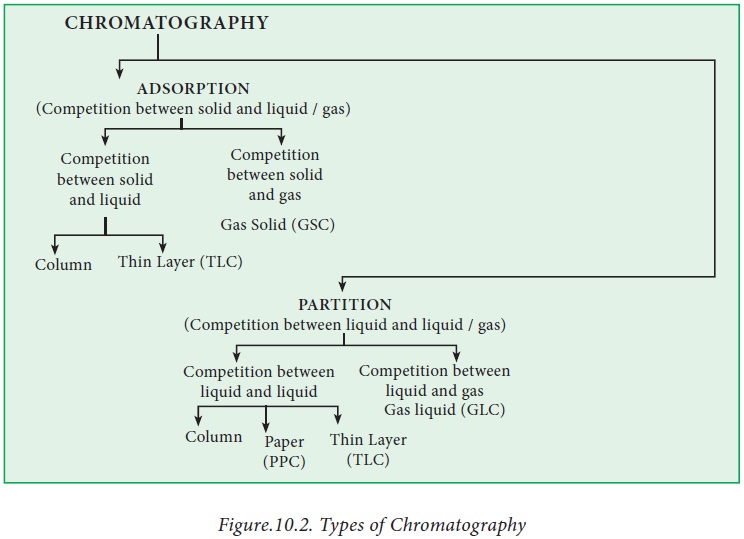
Types of chromatography
Types of chromatography
There are several types of
chromatography which are classified based on the interaction of the sample with
the mobile and stationary phase. In general, the different chromatographic
techniques fall under two major categories: Partition chromatography and adsorption
chromatography. Partition chromatography involves partition between two
liquids. The stationary phase is a liquid and is held in an inert supporting
liquid. In adsorption chromatography, the stationary phase is a finely divided
adsorbent such as silica gel and the mobile phase can be a gas or more commonly
a liquid. The different forms of chromatography are represented in Fig 10.2
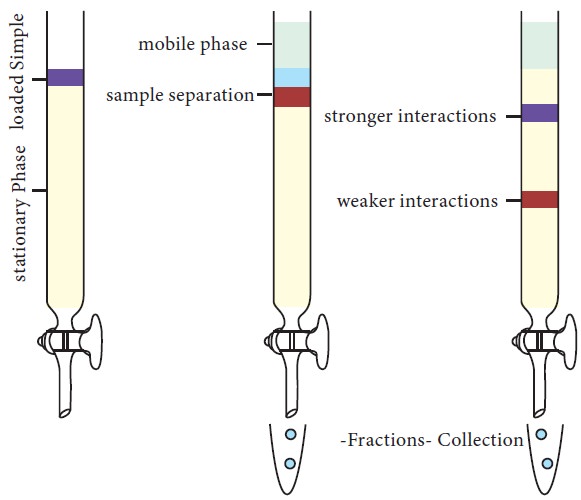
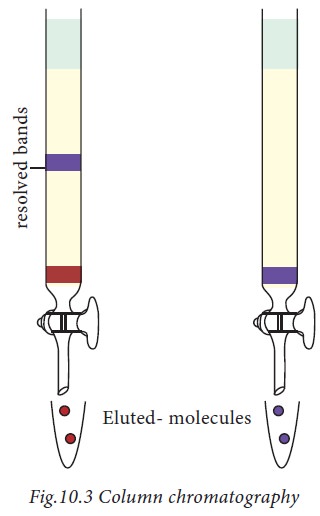
1. Column chromatography
In column chromatography, a mixture
of substances, in solution is applied to a column filled with a permeable solid
matrix immersed in a solvent. As shown in Fig.10.3, sample, for example, a
mixture of protein is applied to the column matrix (packed into a glass or
metal surface). After equilibration, the proteins are eluted out using a specific
mobile phase. Since different proteins are retarded to different extents by the
interaction with the matrix, they can be collected separately. According to the
choice of the matrix, proteins can be separated based on the charge,
hydrophobicity, size or ability to bind to a characteristic group.

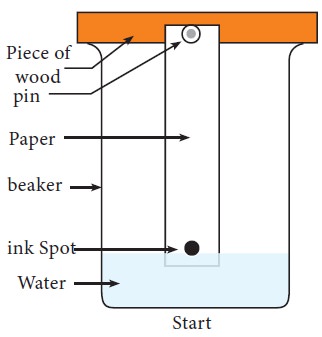
2. Paper chromatography
Paper
chromatography was discovered by the Russian investigators Izmailov and
Schraiber. The filter paper strips, made up of cellulose are used to support
the stationary phase (water) while the mobile organic phase moves on the
suspended paper strip in a chamber. In this technique, the solution containing
the mixture of compounds is spotted at a point above 3cm from the end of a
Whatmann filter paper (Fig.10.4). After spotting the sample, the paper is dried
and equilibrated into a glass jar which contains the desired solvent, for
example butanol: acetic acid: water in the ratio of 4:1:5, which acts as a
mobile phase. It should be ensured that the point of application remains well
above the level of the solvent in the jar. The paper is suspended in such a
manner so that it hangs freely without touching the sides of the container. The
solvent ascends on the paper and is called as ascending chromatography. The
factor governing the separation of compounds in the sample depends on the
partition between two immiscible phases.
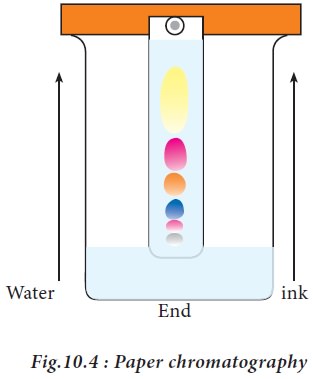
When the
solvent reaches the top of the paper, the paper is removed and the distance to
which the solvent travelled is marked. In order to locate the compounds, the
paper strip is dried and sprayed with a specific developer. For
example, to identify amino acids the strip is sprayed with 0.5% ninhydrin
dissolved in acetone to visualize the colored spots indicating a particular
amino acid. The ratio of the distance travelled by a component (i.e. amino
acid) to that travelled by the solvent front, both measured from the marked
point of application of the mixture, is called the resolution front or
retardation factor (Rf) value for that component. Thus,

3. Adsorption chromatography
Adsorption is the process through
which some substances physically bind to the surface of a solid substance like
charcoal. Mobile phase is either a liquid (liquid – solid chromatography) or
gas (gas-solid chromatography). Adsorption chromatography is based on the
interaction between the solute molecules and active sites on the stationery
phase. If stationary phase is much polar than the mobile phase, high polar
compounds in the sample will bind tightly to the stationary phase while less
polar compounds will bind less to the stationary phase. Therefore, the less
lightly bound compound will be eluted out by the mobile phase earlier than the
one that binds tightly.
4. Thin layer chromatography
Principle: Thin layer chromatography
(TLC) is adsorption chromatography performed on adsorbent materials supported
on glassplates. This technique combines the advantages of paper chromatography
with those of column chromatography. In TLC, the stationery phase (alumina
powder or silica gel with calcium sulphate as binder) is coated on a glass,
plastic or foil plates. The thin layer is air dried at room temperature and
activated in a hot air oven at 100 - 250°C. The mixture to be separated is
spotted on the plate at one end and kept vertical in a container with mobile
phase. After a few minutes, the components are separated through the thin layer
and dried. The spots are detected by spraying the plates with specific staining
reagents.
TLC is superior to paper Chromatography for the following reasons:
· Concentrated sulfuric acid spray followed by heating may be used
to develop the chromatogram by charring, which makes the spots of organic
compounds visible.
· The time taken to separate a mixture of amino acids by paper
chromatography can be reduced by TLC.
· The choice of adsorbents to allow separation of components is
restricted in paper chromatography. In TLC, a wide range of adsorbents can be
used.
5. Ion-exchange chromatography
Principle
: Ion exchange
chromatography was developed by D’Alelio in 1942 using synthetic ion exchange medium based on polystyrene
resin. Ion exchange chromatography is a displacement technique in which the
ions in either the sample or the buffer displace the existing mobile ions associated
with the fixed resin ions. Ion-exchange resins are insoluble, porous matrix
containing numbers of ionic groups capable of binding ions of an opposite
charge from the surrounding solution. As described in Fig.10.5, proteins move
through the column depending upon the net charge at the pH being used.
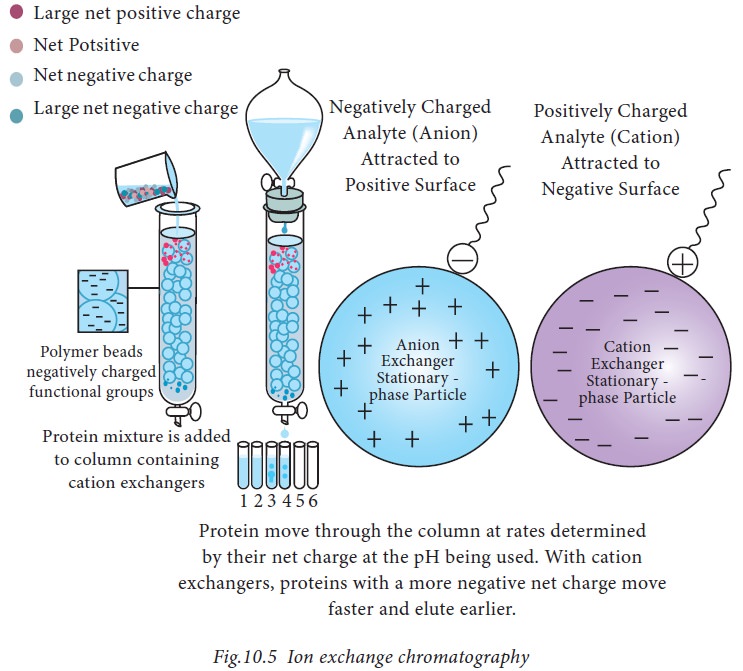
When the
proteins are attached to the column material, increasing salt concentration or
buffers with different pH are passed through the column. Proteins will be
washed of the column at different times depending on their molecular structure,
net charge and side group. Anion exchangers contain immobilized cationic groups
that bind to anions and vice-versa. Examples of anion exchangers are
Diethylaminoethyl (DEAE), aminoethyl (AE). Cationic exchangers include
carboxymethyl (CM) cellulose, sulphopropyl (SP).
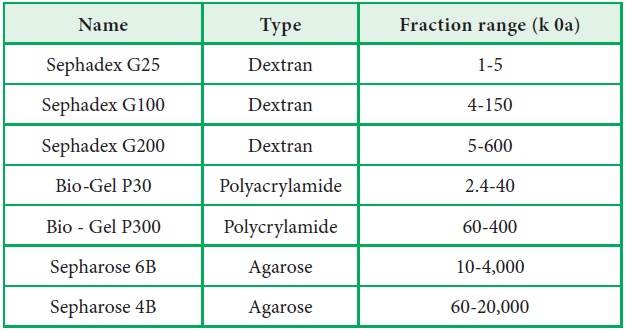
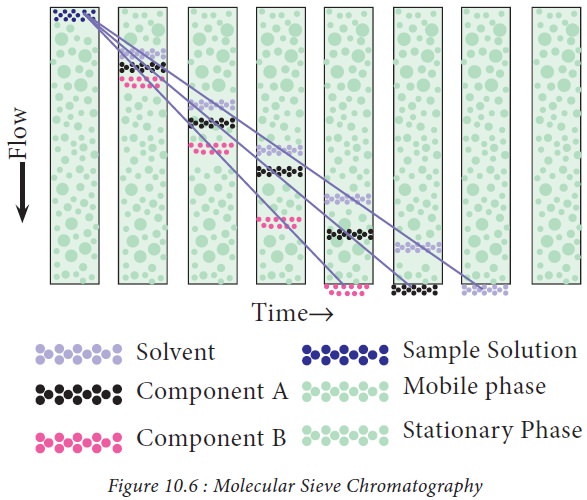
6. Molecular sieve chromatography
Principle
: Gel
permeation or molecular size exclusion chromatography is based on the separation of molecules depending on the molecular
size and shape. It employs column which contains pores of varying diameter.
When the sample is added to the column, molecules of larger size travel faster
than the smaller molecules due to permeation into the gel pores. Therefore,
larger molecules are eluted first from the column, followed by molecules of
smaller size (Fig. 10.6). The gel materials used in gel permeation
chromatography are shown in Figure.10.6.
7. Affinity chromatography
Affinity
chromatography is an efficient technique, where separation of a biomolecule can
be achieved through highly specific interactions. The interaction is based on
the chemical nature of the molecule present in the sample.
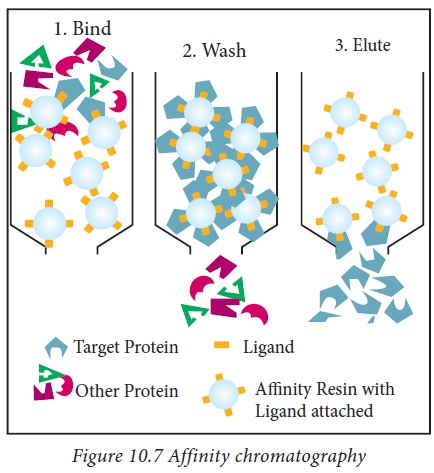
When a
complex mixture containing the specific compound to be purified is added to the
immobilized ligand, only the specific compound will bind reversibly to the
ligand leaving the other constituents. The target compound (protein) can be
suitably recovered by displacement from the ligand using selective buffer with
altered pH (Figure.10.7).
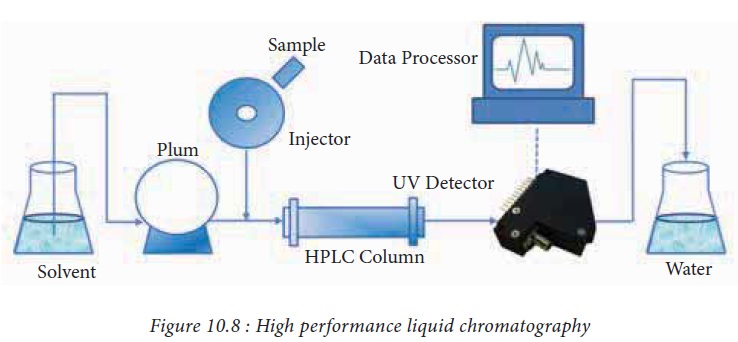
8. High performance liquid chromatography
HPLC
(High Performance Liquid Chromatography) is a highly improved form of liquid
column chromatography used to separate the components of a mixture. Columns
used in the HPLC system are generally made in stainless steel to withstand the
high pressures. Sample administration is through an automated injection system.
Mobile phase (mixture of polar or non-polar liquid components) are contained in
a glass reservoir. Eluents used for HPLC must be purified. Pumping systems for
delivery of the eluent are one of the most important features of HPLC systems.
A detector is used to retrieve the data and its analysis in the form of
chromatogram (Fig.10.8). Applications of HPLC include isolation and purification
of biologically active compounds, purification of chemical compounds, and
developing processes for synthesizing chemical compounds.
Related Topics