Chapter: 11th Biochemistry : Chapter 10 : Biochemical Techniques
Spectrophotometry
Spectrophotometry
The use
of spectroscopic methods to evaluate bio-molecules continues to be an important
area in Biochemistry. Spectroscopy is the study of the absorption and emission
of radiation by matter. Using spectroscopic methods, it is possible to analyze
the colour developed by the bio-molecule exhibited by the substances that
absorb radiation from the visible region of the spectrum. Measurements of the
intensity and wavelength of the radiation that is either absorbed or emitted
provide the basis for sensitive method of detection and quantification. The study
of functions of the body in both health and diseased states require the
analysis of blood, serum and body fluids. The applications of spectrometry has
produced more diagnostic kits to quantify the constituents present in blood,
tissue, urine and other biological material.
We might
have noticed that the colours of two solutions of the same substance, one a
deep and another lighter colour. When the colour of the solution deepens, it is
an indication that it is more concentrated. This forms the basis of spectrophotometry,
where the intensity of the colour is a direct measure of the amount of the
material present in the solution. Light is a form of electromagnetic radiation.
When it falls on a solution one can expect three changes. a). the light can be
reflected by the substance b). the light can be absorbed by the substance c).
only certain wavelengths can be absorbed and the remaining transmitted. The
absorbance of transmitted light is of prime importance in spectrophotometry.
The observed colour is due to the absorption of a certain wavelength of light
in the visible spectrum and transmittance of the other wavelength.
1. Principle of Beer-Lambert’s law
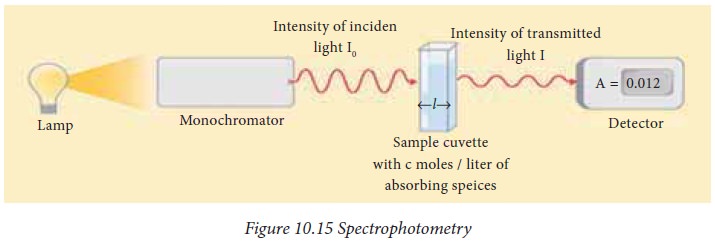
The
absorption of light by a solution is shown in Fig.10.15. When a beam of
monochromatic light (Io) is allowed to pass through a homogenous
light absorbing medium, the intensity of light coming out (I)
decreases exponentially, with increase in the concentration and pathlength of
the medium through which it is passed. The fraction of such incident light
absorbed by a given solution at a particular wave length is related to the
thickness of the absorbing layer i.e., the path length and concentration of the
absorbing species. This relation may be combined using Beer-Lambert’s law to
determine the concentration of analyte in a solution using spectrophotometry.
logIo/I = εcl, where Io and I are the
intensities of incident and transmitted light, respectively. l, is the path
length, c is the concentration of the absorbing species and ε is known as the
molar extinction co-efficient. The expression logIo/I is absorbance.
2. Photo electric colorimeter
Principle:A beam of light
with a precise wavelength is passed through the sample through a mono-chromator and lens, which
navigate the coloured light to the output device which measures the extent of
colour as compared to a standard. A microprocessor then calculates the
absorbance or percent transmittance. If the concentration of the solution is
greater, more light will be absorbed, which can be identified by measuring the
difference between the amount of light at its origin and that after passing
through the solution.
Components of a Colorimeter :
A
schematic representation of the working of the colorimeter is shown in
Fig.10.16
The essential parts of a colorimeter
are: a) light source, which is from a tungsten lamp and the light passes
through a slit b) an aperture which can be adjusted accordingly c) a filter where
the light from the lamp source converts polychromatic light to a monochromatic
light d). photocell or tube which converts the light energy to electrical
energy e) a detector measures the light which was transmitted by the sample f)
cuvette – a glass tube to place the solution.
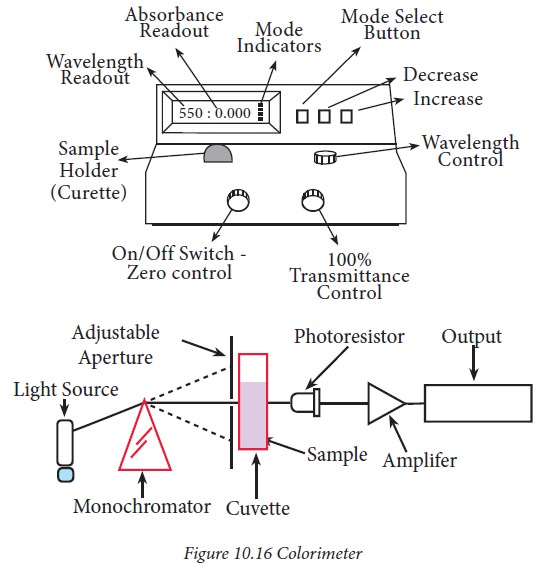
In a
colorimeter, beam of light is passed through an optical filter that transmits
only a particular band of wavelength. The variation in the monochromatic light
transmitted through a colorless sample (blank) and the amount of monochromatic
light transmitted through a test sample is a measurement of the amount of
monochromatic light absorbed by the sample. The monochromatic light absorbed is
directly proportional to the concentration of the sample producing the colour
and the path length through the sample.
Applications
A well
known application of the colorimeter is to determine the concentration of the solute
in a solution. To monitor the growth of a bacterial culture, colorimeter is
used. As the culture grows, the medium becomes cloudier and absorbs more light
which can be quantified. Colorimeters are used for testing water quality and
screening of chemicals.
3. Colorimetric analysis
General
steps in carrying out a photometric analysis is explained using blood glucose
estimation procedure as an example.
· Separation of the substance from the complex
mixture. For example, estimation of blood glucose requires the precipitation of
lipids and proteins using de-proteinising agents which otherwise interfere with
the colour reaction of glucose.
· Qualitative conversion to a coloured or light
absorbing substance. For example, after de-proteinization of the sample, the
supernatant conataining glucose is made to react with orthotoluidine reagent to
give a greenish blue coloured complex.
· Measurement of light absorption of sample. For
example, the colour intensities of the complex can be measured.
· Calculation of the concentration of the substance. Comparing the
molecular extinction with that of a relative standard solution of known
concentration.

4. UV absorption spectrophotometry
Absorption spectrophotometry utilizes
the phenomena of absorption of light by the sample being analysed. Different
materials absorb different wavelengths based on the molecular and chemical
nature of the compound. Absorption spectra can use any form of waves. The
common type of waves used in absorption spectra include infrared, gamma,
atomic, X-ray and visible light. The common wavelength region used in the
absorption spectra is .
Applications of spectrophotometry
·
Spectrophotometry is used in diverse area in the field of biological
science.
·
To identify classes of compounds in pure and biological samples.
·
For quantitative analysis of proteins, lipids and nucleic acid etc.
·
To assay the enzyme activity and their kinetics.
·
Elucidation of the structure of organic compounds.
·
Measurement of growth kinetics.
Related Topics