Chapter: Microbiology and Immunology: Sterilizationand Disinfection
Types of Disinfectants
Types of Disinfectants
Disinfectants include the following: (a) phenolic compounds, (b)
halogens, (c) alcohols, (d) aldehydes, (e) gases, (f) surface
active agents, (g) oxidizing agents,
(h) dyes, (i) heavy metals, and (j)
acids and alkalis.
◗
Phenolic compounds
In 1867, Joseph Lister employed phenolic compounds to reduce the
risk of infection during operations. Phenolic compounds are the most widely
used antiseptics and disinfectants in laboratories and hospitals worldwide.
They are bactericidal or bacteriostatic and some are fungicidal also. They act
by denaturing proteins and disrupting cell membranes. They are effective in the
pres-ence of organic material and remain active on surfaces long after
application. Different phenolic compounds are as follows:
Phenol: It is effective against vegetative forms of bacteria,Mycobacterium tuberculosis, and certain fungi. It is an excellentdisinfectant for feces, blood, pus, sputum, etc. It has a low degree of activity as compared to other derivatives. It is not suitable for application to skin or mucous membrane.
Cresol: Cresols are more germicidal and less poisonousthan phenol but
corrosive to living tissues. They are used for cleaning floors (1% solution),
for disinfection of surgical instruments, and for disinfection of contaminated
objects. Lysol is a solution of cresols in soap.
Halogenated diphenyl compounds: These compoundsinclude
hexachlorophene and chlorhexidine. They are highly effective against both
Gram-positive and Gram-negative bacteria. They are used as skin antiseptics and
for the clean-ing of wound surfaces.
Hexachlorophene has been one of the most popular antiseptics
because once applied it persists on the skin and reduces growth of skin
bacteria for longer periods. However, it can cause brain damage and is now used
in hospital nurseries only after a staphylococcal outbreak.
◗
Halogens
Halogens are fluorine, bromine, chlorine, and iodine—a group of
nonmetallic elements that commonly occur in minerals, sea water, and salts.
Although they can occur either in the ionic (halide) or nonionic state, most
halogens exert their antimicro-bial activity primarily in their nonionic state,
but not in the halide state (e.g., chloride, iodide).
These agents are highly
effective disinfectants and anti-septics, because they are microbicidal and not
just microbi-static. They are also sporicidal with longer exposure. For these
reasons, halogens are the active ingredients in nearly one-third of all
antimicrobial chemicals currently marketed. Chlorine and iodine are the only
two routinely used halogens because fluorine and bromine are dangerous to
handle.
Chlorine and its compounds: Chlorine has been used for
dis-infection and antisepsis for approximately 200 years. The major forms used
in microbial control are (a) liquid
and gaseous chlo-rine and (b)
hypochlorites. In solution, these compounds com-bine with water and release
hypochlorous acid (HOCl), which oxidizes the sulfhydryl (S–H) group on the
amino acid cysteine and interferes with disulfide (S–S) bridges on numerous
enzymes. The resulting denaturation of the enzymes is permanent.
Gaseous and liquid chlorine are used almost
exclusively for large-scale disinfection of drinking water, sewage, and
wastewa-ter from sources, such as agriculture and industry. Chlorine kills not
only bacterial cells and endospores but also fungi and viruses. Treatment of
water with chlorine destroys many patho-genic vegetative microorganisms without
unduly affecting its taste. Chlorination at a concentration of 0.6–1.0 part of
chlorine per million parts of water makes water potable and safe to use.
Common household bleach is a weak solution (5%) of sodium
hypochlorite that is used as an all-around disinfectant, deodor-izer, and stain
remover. It is frequently used as an alternative to pure chlorine in treating
water supplies. However, the major limitations of chlorine compounds are that
they are:
a.
Ineffective if used at an alkaline pH,
b.
Less effective in the presence of excess organic matter, and
c.
Relatively unstable, especially if exposed to light.
Iodine and its compounds: Iodine is a pungent black
chemicalthat forms brown-colored solutions when dissolved in water or alcohol.
Iodine rapidly penetrates the cells of microorganisms, where it apparently
disturbs a variety of metabolic functions. It acts by interfering with the hydrogen
and disulfide bonds of proteins (similar to chlorine). It kills all types of
microorgan-isms if optimum concentrations and exposure times are used. Iodine
activity, unlike chlorine, is not as adversely affected by organic matter and
pH. The two primary iodine preparations are free
iodine in solution and iodophors.
Free iodine in solution:Aqueous iodine contains 2% freeiodine in solution and 2.4% sodium
iodide. It is used as a topicalantiseptic before surgery and also occasionally
as a treatment for burnt and infected skin. A stronger iodine solution (5%
iodine and 10% potassium iodide) is used primarily as a disinfectant for
plas-tic items, rubber instruments, cutting blades, and thermometers.
Iodine tincture is a 2%
solution of iodine and sodium iodide in 70% alcohol that can be used in skin
antisepsis. Because iodine can be extremely irritating to the skin and toxic
when absorbed, strong aqueous solutions and tinctures (5–7%) are no longer
considered safe for routine antisepsis.
Iodine tablets are available for disinfecting water during
emer-gencies or for destroying pathogens in impure water supplies.
Iodophors:Iodophors are complexes of
iodine and a neutralpolymer, such as polyvinyl alcohol. This formulation
permits the slow release of free iodine and increases its degree of
pen-etration. These compounds have largely replaced free iodine solutions in
medical antisepsis because they are less prone to staining or irritating
tissues.
·
Betadine, povidone, and isodine are the common iodophor compounds that
contain 2–10% of available iodine. They are used to prepare skin and mucous
membranes for surgery and in surgical hand scrubs.
·
They are also used to treat burns and to disinfect equipments.
·
A recent study has shown that betadine solution is an effective
means of preventing eye infections in newborn infants, and it may replace
antibiotics and silver nitrate as the method of choice.
◗
Alcohols
Alcohols are among the most widely used disinfectants and
antiseptics. They are bactericidal and fungicidal but not spori-cidal. They
have no action against spores and viruses. Ethyl alcohol and isopropyl alcohol
are the two most popular alcohol germicides. They are effective at a
concentration of 60–70% in water. They act by denaturing bacterial proteins and
possibly by dissolving membrane lipids. They are used as skin antisep-tics.
Isopropyl alcohol is used for disinfection of clinical ther-mometers. A 10–15
minute soaking is sufficient to disinfect thermometers. Methyl alcohol is
effective against fungal spores.
◗ Aldehydes
Formaldehyde and glutaraldehyde are the two most commonly used
aldehydes that are used as disinfectants. They are highly reactive molecules
that combine with nucleic and alkylating molecules. They are sporicidal and can
also be used as chemical sterilants.
Formaldehyde: Formaldehyde is usually
dissolved in water oralcohol before use. In aqueous solution, it is
bactericidal, spo-ricidal, and also effective against viruses. Formalin
solution is 40% aldehyde in aqueous solution. It is used to:
§ Preserve fresh tissue
specimens,
§ Destroy anthrax spores in
hair and wool,
§ Prepare toxoids from toxins,
§ Sterilize bacterial vaccines,
and
§ Kill bacterial cultures and
suspensions.
Glutaraldehyde: A 2% buffered solution of
glutaraldehyde isan effective disinfectant. It is less irritating than
formaldehyde and is used to disinfect hospital and laboratory equipments.
Glutaraldehyde usually disinfects objects within time frame of 10 minutes but
may require as long as 12 hours to destroy all spores. Glutaraldehyde is
especially effective against tubercle bacilli, fungi, and viruses. It can be
used for cleaning cysto-scopes and bronchoscopes, corrugated rubber anesthetic
tubes and face masks, plastic endotracheal tubes, metal instruments, and
polythene tubing.
◗
Gases
Various gaseous agents are used for sterilization of large volume
of heat-sensitive disposable items and also instruments. Ethylene oxide,
formaldehyde gas, and betapropiolactone are frequently used gaseous agents.
Ethylene oxide: Ethylene oxide is a colorless
liquid used forgaseous sterilization. It is active against all kinds of
bacteria, spores, and viruses. It kills all types of microorganisms by
inhibiting proteins and nucleic acids. It is both microbicidal and sporicidal.
It is a highly effective sterilizing agent because it rapidly penetrates
packing materials, including plastic wraps. It is used to sterilize disposable
plastic Petri dishes, sutures, syringes, heart-lung machine, respirators, and
dental equip-ments. Ethylene oxide is highly inflammable and carcinogenic.
Extensive aeration of the sterilized materials is necessary to remove residual
ethylene oxide gas, which is toxic.
Formaldehyde gas: The formaldehyde gas is used
for(a) the fumigation of operation
theaters, wards, sick rooms, and laboratories; and (b) the sterilization of instruments and heat-sensitive catheters,
clothing and bedding, furniture, books, etc. The formaldehyde gas is produced
by adding 150 gm of potassium permanganate to 280 mL formalin in 1000 cu ft of
room volume. The room to be sterilized is completely closed and sealed at least
for 48 hours after fumigation with formalin gas. Sterilization is achieved by
condensation of gas on exposed surface. The gas is toxic when inhaled and is
irri-tant to eye, hence its effect should be nullified by exposure to ammonia.
It is highly inflammable and carcinogenic.
Beta-propiolactone: Beta-propiolactone (BPL) is a
condensa-tion product of ketone and formaldehyde. It is active against all
microorganisms and viruses. It is more efficient than formaldehyde for
fumigation purpose. In the liquid form, it has been used to sterilize vaccines
and sera. BPL destroys microorganisms more readily than ethylene oxide but does
not penetrate materials well and may be carcinogenic. For these reasons, BPL
has not been used as extensively as ethylene oxide. Recently, vapor-phase
hydrogen peroxide has been used to decontaminate biological wastes.
◗ Surface active agents
Surface active agents, such as detergents are the substances that
alter energy relationship at interfaces producing a reduction in surface
tension. Detergents are organic mol-ecules that serve as wetting agents and
emulsifiers because they have both polar hydrophilic and nonpolar
hydrophobicends. Due to their amphipathic nature, detergents solubilize and are
very effective cleansing agents. They are different from soaps, which are
derived from fats. Surface active agents are of four types:
Cationic surface active agents: The cationic detergentsare effective disinfectants. Cationic detergents like benzal-konium chloride and cetylpyridinium chloride kill most bacteria but not M. tuberculosis, endospores, or viruses. They do have the advantages of being stable and nontoxic, but they are inactivated by hard water and soap. These are often used as skin antiseptics and also as disinfectants for disinfection of food utensils and small instruments. Qua-ternary ammonium compounds, such as cetrimide are the most popular cationic detergents. They act by disrupting microbial membranes and possibly by denaturing proteins.
Anionic surface active
agents: These include soaps preparedeither from saturated or unsaturated
fatty acids, which act better at acidic pH. The soaps prepared from saturated
fatty acids are more effective against Gram-negative organisms, whereas those
prepared from unsaturated fatty acids are more active against Gram-positive
bacilli and Neisseria.
Nonionic
surface active agents: These are nontoxic andsome of them may even promote the growth of
bacteria.
Amphoteric or ampholytic compounds: These are activeagainst a
wide range of Gram-positive and Gram-negative bacteria and also against a few
viruses. These are known as “Tego” compounds.
◗
Oxidizing agents
This group includes halogens, hydrogen peroxide, potassium
permanganate, and sodium perborate. They are good disinfec-tants and
antiseptics but are less effective in the presence of organic matter. Hydrogen
peroxide, used as 3% solution, is a weak disinfectant. It is useful for
cleaning of the wounds and for mouth wash or gargle. Potassium permanganate is
bacteri-cidal in nature and active against viruses also.
◗
Dyes
The dyes that have been used extensively as skin and wound
antiseptics include (a) acridine dyes
and (b) aniline dyes. The acridine
dyes include acriflavine, euflavine, proflavine, andaminacrine. They
show more activity against Gram-positive bac-teria than against Gram-negative
organisms. They act by interfer-ing with the synthesis of nucleic acids and
proteins in bacterial cells. The yellow acridine dyes, acriflavine and
proflavine, are sometimes used for antisepsis and wound treatment in medi-cal
and veterinary clinics. Aniline dyes (such as gentian
violent, crystal violet, and malachite green) are also more active against
Gram-positive bacteria than against Gram-negative organisms. They are also
effective against various fungi, hence are incorpo-rated into solutions and
ointments to treat fungal skin infec-tions, such as ringworm.
The dyes,
nevertheless, have limited applications because they stain and have a narrow
spectrum of antimicrobial activ-ity. They also have no activity against
tubercle bacilli. Their actions are also inhibited by the presence of organic
matter.
◗
Heavy metals
Soluble salts of mercury, silver, copper, arsenic, and other heavy
metals have antibacterial activity, both bactericidal and bacte-riostatic. They
combine with proteins, often with their sulfhy-dryl groups and inactivate them.
They may also precipitate cell proteins. Silver compounds are widely used as
antiseptics. Silver sulfadiazine is used for burns. Silver nitrate is used as a
prophylactic agent in ophthalmianeonatorum in newborn infants. Copper sulfate
is an effective algicide in lakes and swimming pools. Mercuric chloride is used
as disinfectant. These compounds, however, are increasingly replaced by other
less toxic and more effective germicides.
◗
Acids and alkalis
Acids (such as sulfuric acid, nitric acid, hydrochloric acid, and
benzoic acid) and alkalis (like potassium and sodium hydroxide and ammonium hydroxide)
are germi-cidal in nature. They kill microorganisms by hydrolysis and altering
the pH of the medium. They are rarely used as disinfectants.
Organic acids are
widely used in food preservation because they prevent spore germination and
bacterial and fungal growth, and because they are generally regarded as safe to
eat. Acetic acid, in the form of vinegar, is a pickling agent that inhibits
bacterial growth. Propionic acid is com-monly added into breads and cakes to
retard molds; lactic acid is added to sauerkraut and olives to prevent growth
of anaerobic bacteria, especially the clostridia; and benzoic and sorbic acids
are added to beverages, syrups, and margarine to inhibit yeasts.
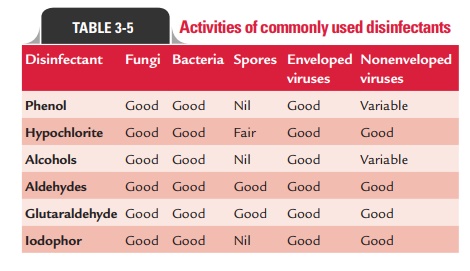
Activities of
commonly used disinfectants against various microorganisms are summarized in
Table 3-5.
Related Topics