Chapter: Microbiology and Immunology: Sterilizationand Disinfection
Physical Methods of Sterilization
Sterilization
Methods of sterilization can be broadly classified as:
a. Physical methods of sterilization, and
b. Chemical methods of sterilization.
Physical Methods of Sterilization
Physical methods of sterilization include the following:
a. Sunlight
b. Heat
c. Filtration
d. Radiation
e. Sound (sonic) waves
◗ Sunlight
Direct sunlight is a natural method of sterilization of water in tanks, rivers, and lakes. Direct sunlight has an active germicidal effect due to its content of ultraviolet and heat rays. Bacteria present in natural water sources are rapidly destroyed by exposure to sunlight.
◗ Heat
Heat is the most dependable method of sterilization and is usually the method of choice unless contraindicated. As a rule, higher temperatures (exceeding the maximum) are microbicidal, whereas lower temperatures (below the minimum) tend to have inhibitory or microbistatic effects. Two types of physical heat are used in sterilization—moist and dry heat.
Sterilization by moist heat
Moist heat occurs in the form of hot water, boiling water, or steam (vaporized water). In practice, the temperature of moist heat usually ranges from 60 to 135°C. Adjustment of pressure in a closed container can regulate the temperature of steam. Moist heat kills microorganisms by denaturation and coagula-tion of proteins. Sterilization by moist heat can be classified as follows:
1. Sterilization at a temperature ,100°C
2. Sterilization at a temperature of 100°C
3. Sterilization at a temperature .100°C
4. Intermittent sterilization
1. Sterilization at a temperature ,100°C: Pasteurization isan example of sterilisation at a temperature ,100°C.
Pasteurization:Fresh beverages (such as milk, fruit juices,beer, and wine) are easily contaminated during collection and processing. Because microbes have potential for spoil-ing these foods or causing illness, heat is frequently used to reduce the microbial load and to destroy pathogens. Pasteurization is a technique in which heat is applied to liq-uids to kill potential agents of infection and spoilage, while at the same time retaining the liquid’s flavor and food value. This technique is named after Louis Pasteur who devised this method. This method is extensively used for steriliza-tion of milk and other fresh beverages, such as fruit juices, beer, and wine which are easily contaminated during collec-tion and processing.
The flash method is preferable for sterilization of milk because it is less likely to change the flavor and nutrient content, and it is more effective against certain resistant pathogens, such as Coxiella and Mycobacterium.
Although pasteurization inactivates most viruses and destroys the vegetative stages of 97–99% of bacteria and fungi, it does not kill endospores or thermoduric species (mostly nonpathogenic lactobacilli, micrococci, and yeasts). Milk is not sterile after regular pasteurization. In fact, it can contain 20,000 microbes per milliliter or more, which explains why even an unopened carton of milk will eventually spoil on prolonged storage. Newer techniques have now been used to produce sterile milk that has a stor-age life of 3 months. In this method, milk is processed with ultrahigh temperature (UHT) of 134°C for 1–2 seconds.
2. Sterilization at a temperature of 100°C: Sterilsation ata temperature of 100ºC includes (a) boiling and (b) steam sterilizer at 100°C.
Boiling:Simple boiling of water for 10–30 minutes killsmost of the vegetative forms of bacteria but not bacterial spores. Exposing materials to boiling water for 30 minutes kills most nonspore-forming pathogens including resis-tant species, such as the tubercle bacillus and staphylo-cocci. Sterilization by boiling is facilitated by addition of 2% sodium bicarbonate to water. Since boiling only once at 100°C does not kill all spores, this method cannot be used for sterilization but only for disinfection. Hence, it is not recommended for sterilizing instruments used for surgical procedure. The greatest disadvantage of this method is that the items sterilized by boiling can be easily recontami-nated when removed from water after boiling.
Steam sterilizer at 100°C:Usually, Koch’s or Arnold’ssteam sterilizer is used for heat-labile substances that tend to degrade at higher temperatures and pressure, such as during the process of autoclaving. These substances are exposed to steam at atmospheric pressure for 90 minutes during which most vegetative forms of the bacteria except for the thermophiles are killed by the moist heat.
3. Sterilization at a temperature > 100°C: This method isotherwise known as sterilization by steam under pressure. A temperature of 100°C is the highest that steam can reach under normal atmospheric pressure at sea level. This pressure is measured at 15 pounds per square inch ( psi), or 1 atmosphere. In order to raise the temperature of steam above this point, it must be pressurized in a closed chamber. This phenomenon is explained by the physical principle that governs the behavior of gases under pres-sure. When a gas is compressed, its temperature rises in direct relation to the amount of pressure. So, when the pressure is increased to 5 psi above normal atmospheric pressure, the temperature of steam rises to 109°C. When the pressure is increased to 10 psi above normal, its tem-perature will be 115°C and at 15 psi (a total of 2 atmo-spheres), it will be 121°C. It is not the pressure by itself that is killing microbes, but the increased temperature it produces. This forms the principle of sterilization by steam under pressure. Such pressure–temperature combi-nations can be achieved only with a special device that can subject pure steam to pressures greater than 1 atmo-sphere. Health and commercial industries use an autoclave for this purpose and a comparable home appliance is the pressure cooker.
Autoclave:It is a cylindrical metal chamber with an airtightdoor at one end and racks to hold materials. The lid is fastened by screw clamp and rendered airtight by an asbestos washer. It has a discharge tap for air and steam at the upper side, a pressure gauge and a safety valve that can be set to blow off at any desired pressure. Heating is usually carried out by electricity. Steam circulates within the jacket and is supplied under pressure to the inner chamber where materials are loaded for sterilization (Fig. 3-1). The water in the autoclave boils when its vapor pressure equals that of surrounding atmosphere. Following the increase of pressure inside the closed vessel, the temperature at which the water boils inside the autoclave also increases. The saturated steam that has a higher penetrative power, on coming in contact with a cooler surface condenses to water and releases its latent heat to that surface. For example, nearly 1600 mL steam at 100°C and at atmospheric pressure condenses into 1 mL of water at 100°C and releases 518 calories of heat. The gross reduction in volume of steam sucks in more steam to the area and this process continues till the temperature of that surface is elevated to that of the steam. Sterilization is achieved when the steam condenses against the objects in the chamber and gradually raises their temperature. The condensed water facilitates moist conditions that ensures killing of microbes.

Sterilization condition: Experience has shown that the mostefficient pressure–temperature combination for achiev-ing sterilization by autoclave is 15 psi, which yields 121°C. It is possible to use higher pressure to reach higher tem-peratures (for instance, increasing the pressure to 30 psi raises the temperature by 11°C), but doing so will not significantly reduce the exposure time and can harm the items being sterilized. It is important to avoid over packing or haphazardly loading the chamber, because it prevents
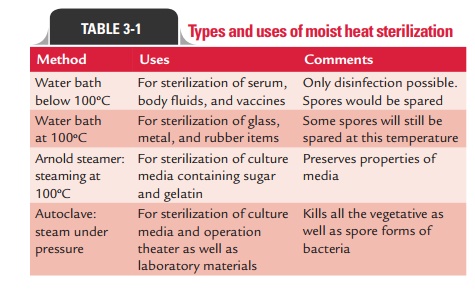
However, the autoclave is ineffective for sterilizing substances that repel moisture (oils, waxes, or powders). Types and uses of various moist heat sterilization methods are summarized in Table 3-1.
Sterilization controls: Various sterilization controls are usedto determine the efficacy of sterilization by moist heat. These include (a) thermocouples, (b) chemical indicators, and (c) bacteriological spores as mentioned below:
a. Thermocouples are used to record temperatures directly in autoclaves by a potentiometer.
b. Brown’s tube is the most commonly used chemical indicator of moist heat sterilization in the autoclave. It contains red solution that turns green when exposed to temperature of 121°C for 15 minutes in an autoclave.
c. Bacillus stearothermophilusspores are used as theindicators of moist heat sterilization in the autoclave. This is a thermophilic bacterium with an optimum temperature of 55–60°C, and its spores require an exposure of 12 minutes at 121°C to be destroyed. The efficacy of the autoclave is carried out by placing paper strips impregnated with 106spores in envelopes and keeping those envelopes in different parts of the load inside the autoclave. These strips after sterilization are inoculated into a suitable recovery medium and
d. Intermittent sterilization: Certain heat-labile substances(e.g., serum, sugar, egg, etc.) that cannot withstand the high temperature of the autoclave can be sterilized by a process of intermittent sterilization, known as tyndallization.
Tyndallizationis carried out over a period of 3 days andrequires a chamber to hold the materials and a reservoir for boiling water. Items to be sterilized are kept in the cham-ber and are exposed to free-flowing steam at 100°C for 20 minutes, for each of the three consecutive days. On the first day, the temperature is adequate to kill all the vegetative forms of the bacteria, yeasts, and molds but not sufficient to kill spores. The surviving spores are allowed to germi-nate to vegetative forms on the second day and are killed on re-exposure to steam. The third day re-ensures killing of all the spores by their germination to vegetative forms.
Intermittent sterilization is used most often to sterilize heat-sensitive culture media, such as those containing sera (e.g., Loeffler’s serum slope), egg (e.g., Lowenstein–Jensen’s medium), or carbohydrates (e.g., serum sugars) and some canned foods.
Sterilization by dry heat
Sterilization by dry heat makes use of air with a low mois-ture content that has been heated by a flame or electric heat-ing coil. In practice, the temperature of dry heat ranges from 160°C to several thousand degrees Celsius. The dry heat kills microorganisms by protein denaturation, oxidative damage, and the toxic effect of increased level of electrolytes. Dry heat is not as versatile or as widely used as moist heat, but it has several important sterilization applications. The temperature and time employed in dry heat vary according to the particular method, but in general they are greater than with moist heat. Sterilization by dry heat includes sterilization by (a) flaming, (b) incineration , and (c) hot air oven:
Flaming: Sterilization of inoculating loop or wire, the tipof forceps, searing spatulas, etc., is carried out by holding them in the flame of the Bunsen burner till they become red hot. Glass slides, scalpels, and mouths of culture tubes are sterilized by passing them through the Bunsen flame without allowing them to become red hot.
Incineration: Incineration is an excellent method forsafely destroying infective materials by burning them to ashes. It has many uses:
Incinerators are used to carry out this process and are regularly employed in hospitals and research labs to destroy hospital and laboratory wastes.
The method is used for complete destruction and dis-posal of infectious material, such as syringes, needles, culture material, dressings, bandages, bedding, animal carcasses, and pathology samples.
This method is fast and effective for most hospital wastes, but not for metals and heat-resistant glass materials.
Hot-air oven: The hot-air oven provides another means ofdry heat sterilization and is the most widely used method. The hot-air oven is electrically heated and is fitted with a fan to ensure adequate and even distribution of hot air in the chamber. It is also fitted with a thermostat that ensures circulation of hot air of desired temperature in the chamber. Heated, circulated air transfers its heat to the materials inside the chamber. While sterilizing by hot-air oven, it should be ensured that the oven is not overloaded. The materials should be dry and arranged in a manner which allows free circulation of air inside the chamber. It is essential to fit the test tubes, flasks, etc., with cotton plugs and to wrap Petri dishes and pipettes in a paper. Steriliza-tion by hot-air oven requires exposure to 160–180°C for 2 hours and 30 minutes, which ensures thorough heating of the objects and destruction of spores (Fig. 3-2).

Thermocouples, chemical indicators, and bacteriological spores of Bacillus subtilis are used as sterilization controls to determine the efficacy of sterilization by hot-air oven.
◗ Filtration
Filtration is an excellent way to reduce the microbial popula-tion in solutions of heat-labile material by use of a variety of filters. Filters are used to sterilize these heat-labile solutions.
Filters simply remove contaminating microorganisms from solutions rather than directly destroying them. The filters are of two types: (a) depth filters and (b) membrane filters.
1. Depth filters: Depth filters consist of fibrous or granularmaterials that have been bonded into a thick layer filled with twisting channels of small diameter. The solution contain-ingmicroorganisms is sucked in through this layer under vacuum and microbial cells are removed by physical screen-ing or entrapment and also by adsorption to the surface of the filter material. Depth filters are of the following types:
Candle filters: These are made up of (a) diatomaceousearth (e.g., Berkefeld filters) or (b) unglazed porcelain (e.g., Chamberlain filters). They are available in differ-ent grades of porosity and are used widely for purifica-tion of water for drinking and industrial uses.
Asbestos filters: These are made up of asbestos such as mag-nesium silicate. Seitz and Sterimat filters are the examples of such filters. These are disposable and single-use discs available in different grades. They have high adsorbing capacity and tend to alkalinize the filtered fluid. Their use is limited by the carcinogenic potential of asbestos.
Sintered glass filters: These are made up of finely pow-dered glass particles, which are fused together. They have low absorbing property and are available in different pore sizes. These filters, although can be cleaned easily, are brittle and expensive.
2. Membrane filters: Membrane filters are made up of (a)cellulose acetate, (b) cellulose nitrate, (c) polycarbonate, (d) polyvinylidene fluoride, or (e) other synthetic materials. These filters are now widely used and have replaced depth filters for last many years. These filters are circular porous membranes and are usually 0.1 mm thick. Although a wide variety of pore sizes (0.015–12 mm) are available, mem-branes with pores about 0.2 mm are used, because the pore size is smaller than the size of bacteria. These filters are used to remove most vegetative cells, but not viruses, from solutions to be filtered. In the process of filtration, the membranes are held in special holders and often preceded by depth filters made of glass fibers to remove larger parti-cles that might clog the membrane filter. The solution is then pushed or forced through the filter with a vacuum or with pressure from a syringe, peristaltic pump, or nitrogen gas bottle, and collected in previously sterilized containers.
Air also can be sterilized by filtration. Two common exam-ples are surgical masks and cotton plugs on culture vessels that let air in but keep microorganisms out. Laminar flow biological safety cabinets are most widely used air filtration systems in hospitals and industries. In this method, air is passed through high-efficiency particulate air (HEPA) filters that remove nearly 99.97% of 0.3 mm particles from the filtered air.
◗ Radiations
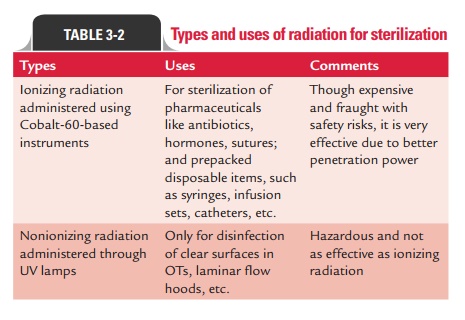
The ionizing and nonionizing radiations are the two types of radiation used for sterilization (Table 3-2).
1. Ionizing radiations: Ionizing radiation is an excellentsterilizing agent with very high penetrating power. These radiations penetrate deep into objects and destroy bacterial endospores and vegetative cells, both prokaryotic and eukaryotic. These are, however, not that effective against viruses. Ionizing radiations include (a) X-rays, (b) gamma rays, and (c) cosmic rays. Gamma radiation from a cobalt-60 source is used for sterilization of antibiotics, hormones, sutures, catheters, animal feeds, metal foils, and plastic disposables, such as syringes. This has also been used to sterilize and “pasteurize” meat and other food items.
Irradiation usually kills Escherichia coli O157:H7, Staphylococcus aureus, Campylobacter jejuni, and other patho-gens. Since there is no detectable increase of temperature in this method, this method is commonly referred to as “cold sterilization.” Both the Food and Drug Administration and the World Health Organization have approved food irradiation and declared it safe.
2. Nonionizing radiations: Nonionizing radiations includeinfrared and ultraviolet radiations.
o Infrared radiations are used for rapid and mass steriliza-tion of disposable syringes and catheters.
o Ultraviolet (UV) radiation with wavelength of 240–280 nmis quite lethal and has a marked bactericidal activity. It acts by denaturation of bacterial protein and also inter-feres with replication of bacterial DNA.
UV radiation is used primarily for disinfection of closed areas in microbiology laboratory, inoculation hoods, laminar flow, and operating theaters. It kills most vegetative bacteria but not spores, which are highly resistant to these radiations. However, it does not penetrate glass, dirt films, water, and other sub-stances very effectively.
Since UV radiations on prolonged exposure tend to burn the skin and cause damage to the eyes, UV lamps should be switched off while people are working in such areas.
◗ Sound (sonic) waves
High-frequency sound (sonic) waves beyond the sensitivity of the human ear are known to disrupt cells. Sonication transmits vibra-tions through a water-filled chamber (sonicator) to induce pres-sure changes and create intense points of turbulence that can stress and burst cells in the vicinity. Sonication also forcefully dislodges foreign matter from objects. Heat generated by the sonic waves (up to 80°C) also appears to contribute to the antimicrobial action.
Gram-negative rods are most sensitive to ultrasonic vibra-tions, while Gram-positive cocci, fungal spores, and bacterial spores are resistant to them. Ultrasonic devices are used in dental and some medical offices to clear debris and saliva from instruments before sterilization and to clean dental restorations. However, most sonic machines are not reliable for regular use in disinfection or sterilization.
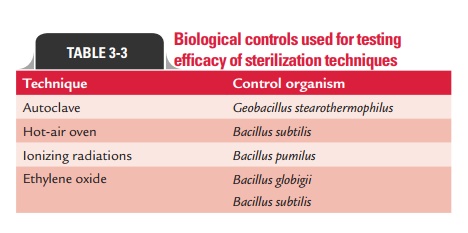
Biological controls used for testing the efficacy of steriliza-tion techniques are summarized in Table 3-3.
Related Topics