Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Sporozoa
Treatment - Malaria
TREATMENT
The indications for treatment rest on two factors. The first is the infecting species of Plas-modium, and the second is the immune status of the afflicted patient. Falciparum malariais potentially lethal in nonimmune individuals such as new immigrants or travelers to a malarious area and immunosuppressed indigenous individuals such as pregnant women. These individuals must be treated emergently.
The complete treatment of malaria requires the destruction of three parasitic forms: the erythrocytic schizont, the hepatic schizont, and the erythrocytic gametocyte. The first termi-nates the clinical attack, the second prevents relapse, and the third renders the patient nonin-fectious to Anopheles and thus breaks the cycle of transmission. Unfortunately, no single drug accomplishes all three goals. The present strategy of chemotherapy is shown in Table 52–2.
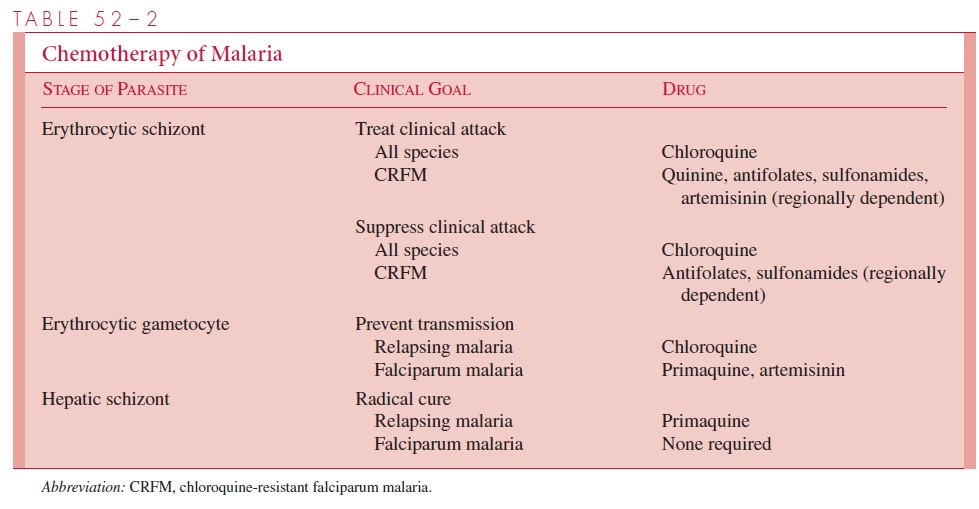
Termination of Acute Attack
Several agents can destroy asexual erythrocytic parasites. Chloroquine, a 4-aminoquinoline, has been the most commonly used. It acts by inhibiting the degradation of hemoglobin,
It has been suggested that the weak basic nature of chloroquine also acts to raise the pH of the food vacuoles of the parasite, inhibiting their acid proteases and effectiveness. When originally introduced, it was rapidly effective against all four species of plasmodia and, in the dosage used, free of serious side effects. However, chloroquine-resistant strains of P. falciparum are now wide-spread in Africa and Southeast Asia; they are also found, although less frequently, in other areas of Asia and in Central America and South America. Other schizonticidal agents in-clude quinine/quinidine, antifolate–sulfonamide combinations, mefloquine, halofantrine, and the artemisinins. Except for the artemisinins, malaria resistance to all of the above agents is increasing. The artemisinins are also unique in their capacity to reduce transmis-sion by preventing gametocyte development.
Strains of P. malariae, P. ovale, and P. vivax (except for some acquired in the South Pacific and South America) remain sensitive to chloroquine and may be treated with this agent. P. vivax infections acquired in New Guinea and Sumatra, however, should be as-sumed to be chloroquine-resistant and managed with mefloquine alone or in combination with other agents. P. falciparum has now become variably resistant to all drug groups ex-cept the artemisinin compounds (see Fig 52–3).
There is a growing consensus that the most effective way to slow the further develop-ment of drug-resistant strains of P. falciparum is to use one of the artemisinins in combination with quinine/quinidine, antifolate–sulfonamide compounds, mefloquine, or halofantrine.
Radical Cure
In P. vivax and P. ovale infections, hepatic schizonts persist and must be destroyed to pre-vent reseeding of circulating erythrocytes with consequent relapse. Primaquine, an 8-aminoquinaline, is used for this purpose. Some P. vivax infections acquired in Southeast Asia and New Guinea fail initial therapy due to relative resistance to this 8-amino-quinaline. Retreatment with a larger dose of primaquine is usually successful. Unfortu-nately, primaquine may induce hemolysis in patients with G6PD deficiency. Persons of Asian, African, and Mediterranean ancestry should thus be screened for this abnormality before treatment. Chloroquine destroys the gametocytes of P. vivax, P. ovale, and P.malariae but not those of P. falciparum. Primaquine and artemisinins, however, are effec-tive for this latter species.
Related Topics