Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Sporozoa
Malaria : Clinical Aspects
MALARIA : CLINICAL ASPECTS
MANIFESTATIONS
The incubation period between the bite of the mosquito and the onset of disease is ap-proximately 2 weeks. With P. malariae and with strains of P. vivax in temperate climates, however, this period is often more prolonged. Individuals who contract malaria while tak-ing antimalarial suppressants may not experience illness for many months. In the United States, the interval between entry into the country and onset of disease exceeds 1 month in 25% of P. falciparum infections and 6 months in a similar proportion of P. vivax cases.
The clinical manifestations vary with the species of plasmodia but typically include chills, fever, splenomegaly, and anemia. The hallmark of disease is the malarial parox-ysm. This manifestation begins with a cold stage, which persists for 20 to 60 minutes. During this time, the patient experiences continuous rigors and feels cold. With the conse-quent increase in body temperature, the rigors cease and vasodilatation commences, ushering in a hot stage. The temperature continues to rise for 3 to 8 hours, reaching a maximum of 40 to 41.7°C before it begins to fall. The wet stage consists of a decrease in fever and profuse sweating. It leaves the patient exhausted but otherwise well until the onset of the next paroxysm.
Typical paroxysms first appear in the second or third week of fever, when parasite sporulation becomes synchronized. In falciparum malaria, synchronization may never take place, and the fever may remain hectic and unpredictable. The first attack is often severe and may persist for weeks in the untreated patient. Eventually the paroxysms be-come less regular, less frequent, and less severe. Symptoms finally cease with the disap-pearance of the parasites from the blood.
In falciparum malaria, capillary blockage can lead to several serious complications. When the central nervous system is involved (cerebral malaria), the patient may develop delirium, convulsions, paralysis, coma, and rapid death. Acute pulmonary insufficiency fre-quently accompanies cerebral malaria, killing about 80% of those involved. When splanchnic capillaries are involved, the patient may experience vomiting, abdominal pain, and diarrhea with or without bloody stools. Jaundice and acute renal failure are also common in severe ill-ness. These pernicious syndromes generally appear when the intensity of parasitemia ex-ceeds 100,000 organisms per cubic millimeter of blood. Most deaths occur within 3 days.
DIAGNOSIS
Malarial parasites can be demonstrated in stained smears of the peripheral blood in virtu-ally all symptomatic patients. Typically, capillary or venous blood is used to prepare both thin and thick smears, which are stained with Wright or Giemsa stain and examined for the presence of erythrocytic parasites. Thick smears, in which erythrocytes are lysed with water before staining, concentrate the parasites and allow detection of very mild para-sitemia. Nonetheless, it may be necessary to obtain several specimens before parasites are seen. Artifacts are numerous in thick smears, and correct interpretation requires experi-ence. The morphologic differences among the four species of plasmodia allow their speci-ation on the stained smear by the skilled observer.
A number of attempts have been made to improve on the standard thin and thick smear. One such procedure involves acridine orange staining of centrifuged parasites in quantitative buffy coat (QBC) tubes. Although it is expensive, requires a fluorescence mi-croscope, and permits less reliable parasite speciation, its rapidity and ease of use make it attractive to laboratories that are only occasionally called on to identify patients with malaria. Simple, specific card antigen detection procedures are now available. The most widely used test, ParaSight F, detects a protein (HRP2) excreted by P. falciparum within minutes. The test can be performed under field conditions and has a sensitivity more than 95%. A second rapid test, OptiMAL, detects parasite lactate dehydrogenase, and, unlike ParaSight F, can distinguish between P. falciparum and P. vivax. Serologic tests for malaria are offered at a few large reference laboratories but are used primarily for epi-demiologic purposes. They are occasionally helpful in speciation and detection of other-wise occult infections. The recently completed sequencing of the malaria genome will lead to newer diagnostic methods.
TREATMENT
The indications for treatment rest on two factors. The first is the infecting species of Plas-modium, and the second is the immune status of the afflicted patient. Falciparum malariais potentially lethal in nonimmune individuals such as new immigrants or travelers to a malarious area and immunosuppressed indigenous individuals such as pregnant women. These individuals must be treated emergently.
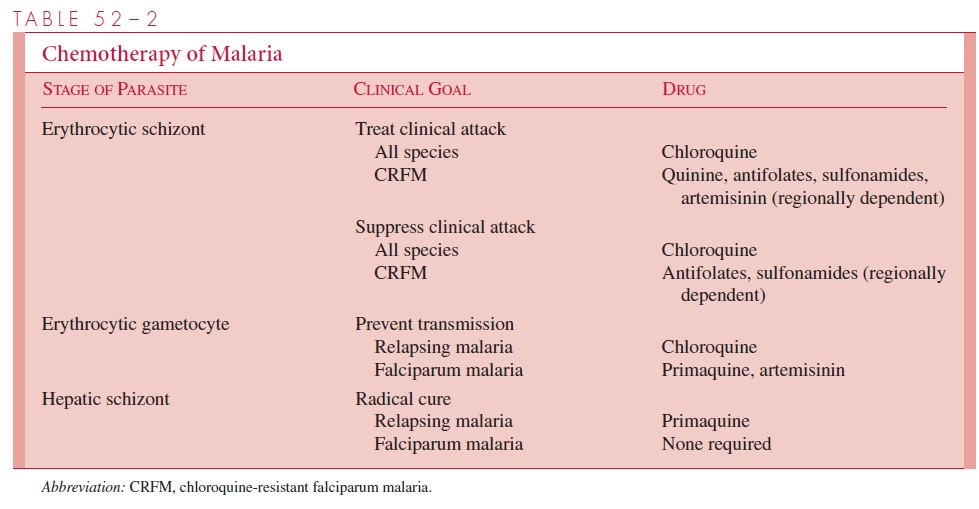
The complete treatment of malaria requires the destruction of three parasitic forms: the erythrocytic schizont, the hepatic schizont, and the erythrocytic gametocyte. The first termi-nates the clinical attack, the second prevents relapse, and the third renders the patient nonin-fectious to Anopheles and thus breaks the cycle of transmission. Unfortunately, no single drug accomplishes all three goals. The present strategy of chemotherapy is shown in Table 52–2.
Termination of Acute Attack
Several agents can destroy asexual erythrocytic parasites. Chloroquine, a 4-aminoquinoline, has been the most commonly used. It acts by inhibiting the degradation of hemoglobin,
thereby limiting the availability of amino acids necessary for growth. It has been suggested that the weak basic nature of chloroquine also acts to raise the pH of the food vacuoles of the parasite, inhibiting their acid proteases and effectiveness. When originally introduced, it was rapidly effective against all four species of plasmodia and, in the dosage used, free of serious side effects. However, chloroquine-resistant strains of P. falciparum are now wide-spread in Africa and Southeast Asia; they are also found, although less frequently, in other areas of Asia and in Central America and South America. Other schizonticidal agents in-clude quinine/quinidine, antifolate–sulfonamide combinations, mefloquine, halofantrine, and the artemisinins. Except for the artemisinins, malaria resistance to all of the above agents is increasing. The artemisinins are also unique in their capacity to reduce transmis-sion by preventing gametocyte development.
Strains of P. malariae, P. ovale, and P. vivax (except for some acquired in the South Pacific and South America) remain sensitive to chloroquine and may be treated with this agent. P. vivax infections acquired in New Guinea and Sumatra, however, should be as-sumed to be chloroquine-resistant and managed with mefloquine alone or in combination with other agents. P. falciparum has now become variably resistant to all drug groups ex-cept the artemisinin compounds (see Fig 52–3).
There is a growing consensus that the most effective way to slow the further develop-ment of drug-resistant strains of P. falciparum is to use one of the artemisinins in combination with quinine/quinidine, antifolate–sulfonamide compounds, mefloquine, or halofantrine.
Radical Cure
In P. vivax and P. ovale infections, hepatic schizonts persist and must be destroyed to pre-vent reseeding of circulating erythrocytes with consequent relapse. Primaquine, an 8-aminoquinaline, is used for this purpose. Some P. vivax infections acquired in Southeast Asia and New Guinea fail initial therapy due to relative resistance to this 8-amino-quinaline. Retreatment with a larger dose of primaquine is usually successful. Unfortu-nately, primaquine may induce hemolysis in patients with G6PD deficiency. Persons of Asian, African, and Mediterranean ancestry should thus be screened for this abnormality before treatment. Chloroquine destroys the gametocytes of P. vivax, P. ovale, and P.malariae but not those of P. falciparum. Primaquine and artemisinins, however, are effec-tive for this latter species.
PREVENTION
Personal Protection
In endemic areas, mosquito contact can be minimized with the use of house screens, in-secticide bombs within rooms, and/or insecticide-impregnated mosquito netting around beds. Those who must be outside from dusk to dawn, the period of mosquito feeding, should apply insect repellent and wear clothing with long sleeves and pants. In addition, it is possible to suppress clinical manifestations of infection, should they occur, with a weekly dose of chloroquine. In areas where chloroquine-resistant strains are common, an alternative schizonticidal agent should be used. Mefloquine or doxycycline are usually preferred. The antifolate pyrimethamine plus a sulfonamide can be taken as well. How-ever, use of this combination is occasionally accompanied by serious side effects, so it is recommended only when mefloquine- and doxycycline-resistant strains are present in the area, and then only for individuals residing in areas of intense transmission for prolonged periods of time. On leaving an endemic area, it is necessary to eradicate residual hepatic parasites with primaquine before discontinuing suppressive therapy.
General
Malaria control measures have been directed toward reducing the infected human and mosquito populations to below the critical level necessary for sustained transmission of disease. The techniques employed include those mentioned previously, treatment of febrile patients with effective antimalarial agents, chemical or physical disruption of mos-quito breeding areas, and use of residual insecticide sprays. An active international coop-erative program aimed at the eradication of malaria resulted in a dramatic decline in the incidence of the disease between 1956 and 1968. Eradication was not achieved, however, because mosquitoes became resistant to some of the chemical agents used, and today malaria still infects 200 to 300 million inhabitants of Africa, Latin America, and Asia. Tropical Africa alone accounts for 100 million of the afflicted and for most of the 1 to 3 million deaths that occur annually as a result of this disease. The long-term hope for progress in these areas now depends on the development of new technologies.
Vaccines
Three advances in the last decade have produced the hope that an effective malaria vac-cine might be within reach of medical science for the first time. The establishment of a continuous in vitro culture system provided the large quantities of parasite needed for antigenic analysis. Development of the hybridoma technique allowed the preparation of monoclonal antibodies with which antigens responsible for the induction of protective immunity could be identified. Finally, recombinant DNA procedures enabled scientists to clone and sequence the genes encoding such antigens, permitting the amino acid structure to be determined and peptide sequences suitable for vaccine development to be identified.
As immunity to malaria is stage specific, the relative advantages and disadvantages of vaccines prepared against each of the plasmodial stages found in the human host (sporozoite, merozoite, and gametocyte) need to be considered. An effective sporozoite vaccine, by blocking the invasion of hepatocytes by mosquito-introduced sporozoites, would prevent the establishment of the infection within the host and, if widely adminis-tered, would interrupt parasite transmission within a community. However, to be effec-tive, a sporozoite vaccine would have to prevent the invasion of all injected sporozoites. Theoretically, if even a single parasite reached and penetrated a liver cell, it would mul-tiply intracellularly and later enter the bloodstream to invade erythrocytes. The patient could develop clinical disease and serve as a reservoir for subsequent transmission to others. A vaccine directed at the erythrocytic or merozoite stage, although preventing neither hepatic nor bloodstream infection, would limit the severity of the parasitemia and thus moderate or abort clinical manifestations of disease. Gametogenesis, and thus parasite transmission, would probably proceed unimpaired. Antibodies formed in re-sponse to a gametocyte vaccine might block the union of male and female gametes within the mosquito gut, interrupting parasite transmission. It would, however, neither prevent nor moderate malaria in the immunized patient. The limitations of each vaccine type has led some investigators to advocate the combination of all three in a single poly-valent preparation. Unfortunately, the results of field tests of a number of candidate vac-cines have been disappointing.
Related Topics