Chapter: Essentials of Anatomy and Physiology: The Respiratory System
Transport of Gases in the Blood
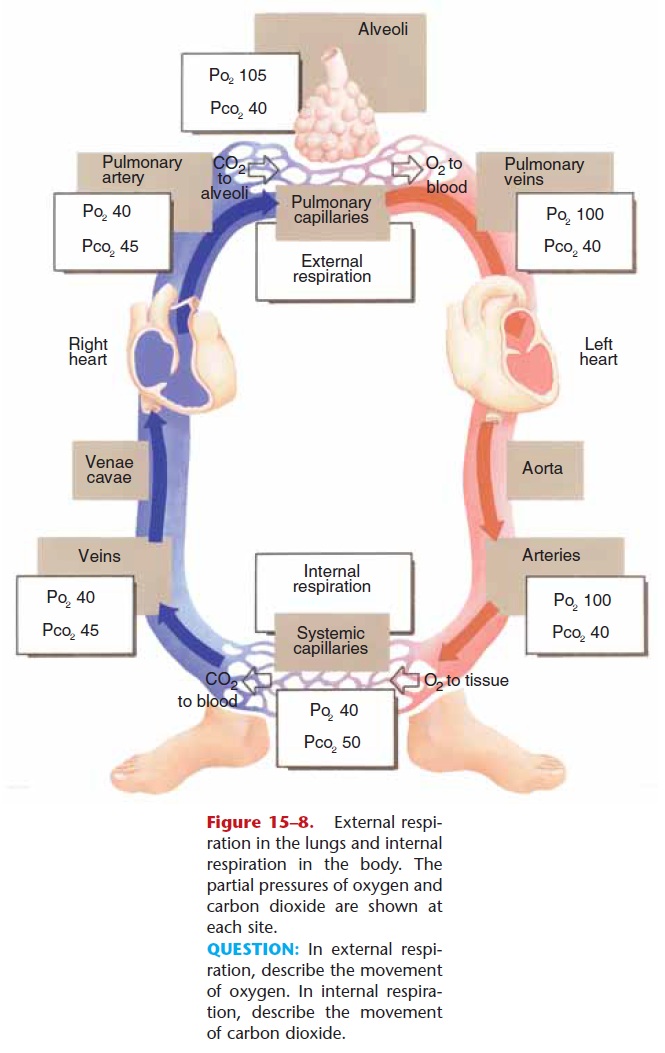
TRANSPORT OF GASES IN THE BLOOD
Although some oxygen is dissolved in blood plasma and does create the PO2 values, it is only about 1.5% of the total oxygen transported, not enough to sustain life. As you already know, most oxygen is carried in the blood bonded to the hemoglobin in red blood cells (RBCs). The mineral iron is part of hemoglobin and gives this protein its oxygen-carrying ability.
The oxygen–hemoglobin bond is formed in the lungs where PO2 is high. This bond, however, is rela-tively unstable, and when blood passes through tissues with a low PO2, the bond breaks, and oxygen is released to the tissues. The lower the oxygen concen-tration in a tissue, the more oxygen the hemoglobin will release. This ensures that active tissues, such as exercising muscles, receive as much oxygen as possible to continue cell respiration. Other factors that increase the release of oxygen from hemoglobin are a high PCO2(actually a lower pH) and a high temperature, both of which are also characteristic of active tissues.
Another measure of blood oxygen is the percent of oxygen saturation of hemoglobin (SaO2). The higher the PO2, the higher the SaO2, and as PO2 decreases, so does SaO2, though not as rapidly. A PO2 of 100 is an SaO2 of about 97% , as is found in systemic arteries. A PO2 of 40, as is found in systemic veins, is an SaO2 of about 75%. Notice that venous blood still has quite a bit of oxygen. Had this blood flowed through a very active tissue, more of its oxygen would have been released from hemoglobin. This venous reserve of oxygen provides active tissues with the oxygen they need.
Carbon dioxide transport is a little more compli-cated. Some carbon dioxide is dissolved in the plasma, and some is carried by hemoglobin (carbaminohemo-globin), but these account for only about 20% of total CO2 transport. Most carbon dioxide is carried in the plasma in the form of bicarbonate ions (HCO3–). Let us look at the reactions that transform CO2 into a bicarbonate ion.
When carbon dioxide enters the blood, most dif-fuses into red blood cells, which contain the enzyme carbonic anhydrase. This enzyme (which contains zinc) catalyzes the reaction of carbon dioxide and water to form carbonic acid:
CO2 + H2O → H2CO3
The carbonic acid then dissociates:
H2CO3 → H+ + HCO3–
The bicarbonate ions diffuse out of the red blood cells into the plasma, leaving the hydrogen ions (H+) in the red blood cells. The many H+ ions would tend to make the red blood cells too acidic, but hemoglobin acts as a buffer to prevent acidosis. To maintain an ionic equilibrium, chloride ions (Cl–) from the plasma enter the red blood cells; this is called the chloride shift. Where is the CO2? It is in the plasma as part of HCO3– ions. When the blood reaches the lungs, an area of lower PCO2, these reactions are reversed, and CO2is re-formed and diffuses into the alveoli to be exhaled.
Related Topics