Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Hematopoietic Growth Factors
Toxicities
TOXICITIES
Many HGFs, especially multipotential factors that act on early
progenitor cells, are associated with constitu-tional symptoms, such as fever,
chills, rash, myalgia, injection-site reaction, and edema. The safety of
individual HGFs depends on their receptor sites and the effects of secondary
cytokine release.
Determination of the relative toxicity of HGFs is difficult because of the lack of comparative studies and confounding effects of different reporting methods and different cancer chemotherapy regimens. For example, patients who undergo bone marrow transplantation experience toxicity that may obscure HGF-related adverse effects. Formulations with different levels of glycosylation may also play a role.
Because these products are recombinant proteins, a small risk of developing antibodies is possible. Development of neutralizing antibodies required the cessation of clinical development of MGDF and, more recently, a form of rhEPO caused a sudden spike in the number of patients who developed pure red cell aplasia (Casadevall et al., 2002; Gershon et al., 2002).
All drugs that are effective have inherent adverse effects, but
compliance with guidelines and dosage regimens approved by regulatory
authorities can lessen untoward effects. Use of rhEPO in treatment regimens
outside the currently approved labeling and guidelines increased mortality in patients
with head-and-neck cancer (Henke et al, 2003; Leyland-Jones and Mahmud, 2004.
Unfortunately, abuse of rhEPO by athletes is another problem (Catlin et al.,
2003).
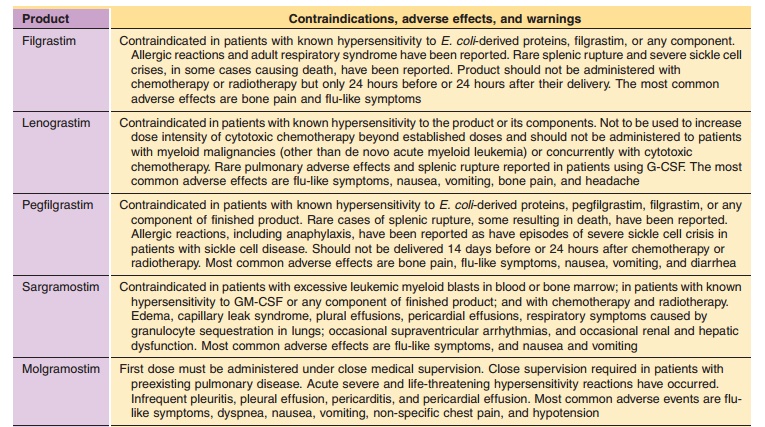
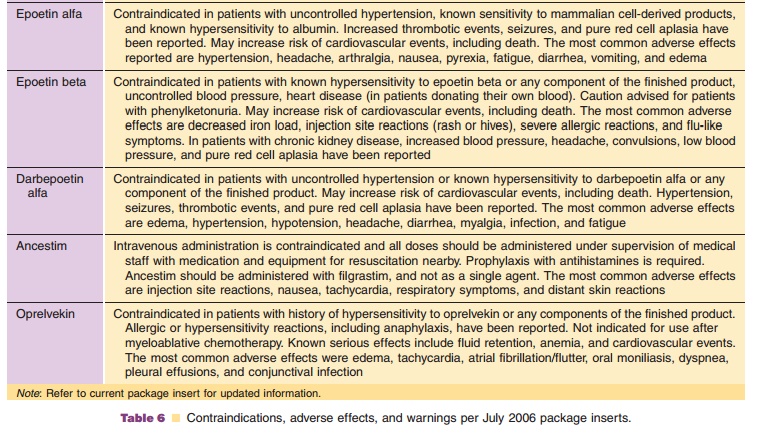
Again, the healthcare provider is responsible for consulting the current
product package insert of all medicines for precautions, warnings, and possible
drug interactions. A summary of the most common adverse events is presented
(Table 6), as listed in the current package inserts, but it should not be
con-sidered to be comprehensive or inclusive.
Related Topics