Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Hematopoietic Growth Factors
Established Uses - Hematopoietic Growth Factors
ESTABLISHED USES
It is impossible to review all literature that explores the investigational use of HGFs. Thus, only the pivotal studies for each established use have been selected to illustrate the benefit of HGFs in patients with hematologic disorders.
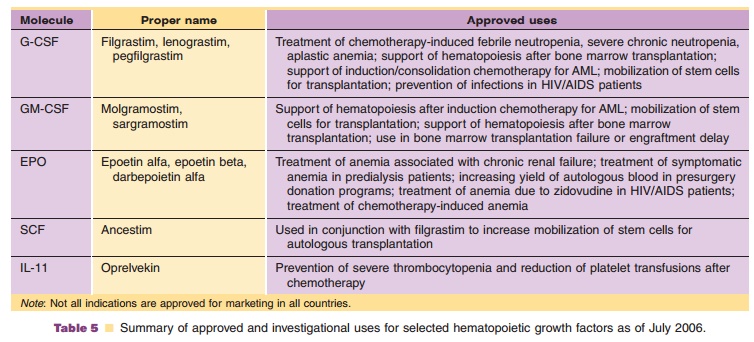
Not all uses discussed here have received regulatory approval in all countries (Table 5). Refer to the current product package insert for licensed indications in the country of interest.
Chemotherapy-Induced Neutropenia
Neutropenia and infection are common dose-limiting effects of cancer chemotherapy. The risk of infection is directly related to the depth and duration of neu-tropenia. The severity of neutropenia depends on the intensity of the cancer chemotherapy regimen, as well as host- and disease-related factors. Fever may be the only manifestation of infection because underlying immunosuppression often obscures the classic signs and symptoms. Therefore, it is standard practice to administer broad-spectrum antibiotic therapy and even to hospitalize patients who have febrile neutro-penia. Furthermore, oncologists may delay the start of subsequent cycles of chemotherapy until neutrophil recovery, decrease the dose of cancer chemotherapy, or both. Although this practice may be deemed necessary to prevent infectious complications, it may also compromise otherwise effective cancer chemotherapy.
Filgrastim
The phase III pivotal trials for filgrastim demon-strated beneficial effects of the HGF on febrile neutropenia after standard-dose chemotherapy (Crawford et al., 1991; Trillet-Lenoir et al., 1993). In these two randomized, double-blind, placebo-con-trolled trials involving > 300 patients with small-cell lung cancer, filgrastim significantly decreased the incidence, severity, and duration of neutropenia, days of hospitalization and duration of intravenous anti-biotic use.
Lenograstim
In phase III studies, prophylactic administration of lenograstim shortened the duration of chemotherapy-induced neutropenia in patients with non-myelogen-ous cancers who received standard-dose chemother-apy or myeloablative chemotherapy followed by bone marrow transplantation (Gisselbrecht et al., 1994; Chevallier et al., 1995). The median neutrophil nadir was significantly higher in patients treated with lenograstim compared with placebo recipients. Incidences of culture-confirmed infections across all cycles were significantly reduced in the lenograstim group during the period of neutropenia. The inci-dence of all infections in lenograstim-treated patients was lower than in the placebo group, but the difference was not statistically significant.
Pegfilgrastim
Two double-blind, active-controlled, phase III studies established the utility of pegfilgrastim in patients with cancer who were treated with doxorubicin and docetaxel (Holmes et al., 2002; Green et al., 2003). In the Holmes et al. study, 310 patients were randomly assigned to receive on day 2 of chemotherapy either a single dose of pegfilgrastim or daily injections of filgrastim. The results of the study showed that one dose of pegfilgrastim per chemotherapy cycle was comparable to daily injections of filgrastim in terms of neutrophil nadir and recovery. The Green et al. study used a fixed dose of pegfilgrastim, again compared with daily doses of filgrastim. In this study with 157 patients, one dose of pegfilgrastim per chemotherapy cycle was comparable to daily injections of filgrastim.
Sargramostim
Sargramostim is not indicated for the reduction of chemotherapy-induced neutropenia, but it isindicated for use after induction chemotherapy in adults aged > 55 years with acute myeloid leukemia to shorten time to neutrophil recovery and to reduce the incidence of severe and life-threatening infections. In a phase III multicenter, randomized, double-blind, placebo-controlled study, sargramostim significantly shortened the median duration of neutropenia after induction chemotherapy compared with controls (Rowe et al., 1995). During consolidation chemotherapy, however, the use of sargramostim did not shorten the median time to recovery of neutrophils com-pared with placebo. The incidence of severe infec-tions and deaths associated with infections was significantly reduced in patients who received sargramostim compared with those who received placebo.
Molgramostim
In a phase III trial, patients with high-grade non-Hodgkin’s lymphoma were administered molgramos-tim as a subcutaneous injection for 7 days after chemotherapy (Gerhartz et al., 1993). The frequency of infections, periods of neutropenia, days with fever, and days of hospitalization for infection were reduced significantly, and white blood cell counts increased.
Bone Marrow or Stem Cell Transplantation
Bone marrow transplantation allows for the use of very high doses of chemotherapy, with or without radiotherapy, to eliminate malignant cells from patients with refractory tumors. The procedure involves administering ablative cancer chemotherapy and then infusing bone marrow progenitor cells that were harvested from the patient (autologous trans-plantation) or a donor (allogeneic transplantation). Before the patient’s bone marrow recovers fullfunction, the neutrophil count usually drops to zero and most patients experience profound pancytopenia and require multiple transfusions of blood and blood products. Allogeneic transplantation is more compli-cated than autologous transplantation because donor white blood cells may recognize host antigens as foreign and attack host tissues. Graft-versus-host disease can be life-threatening and is manifested by epithelial damage in the skin, liver, and gastrointest-inal tract. Consequently, recipients of allogeneic transplantation receive immunosuppressive therapy, which further increases the risk of infection. Regardless of the source of the bone marrow, the procedure may necessitate prolonged hospitalization, which increases the cost of treatment.
Filgrastim
Two randomized, controlled trials have shown the utility of filgrastim in the autologous bone marrow transplantation setting. In one study, filgrastim re-duced the median number of days of severe neutro-penia compared with placebo (Schmitz et al., 1995). The other showed a statistically significant reduction in the median number of days of severe neutropenia in filgrastim-treated patients and the number of days of febrile neutropenia was reduced significantly, as well (Stahel et al., 1994).
Lenograstim
A phase III trial of 315 patients demonstrated the beneficial effects of lenograstim on neutrophil recov-ery in patients receiving either autologous or allo-geneic bone marrow transplantation (Gisselbrecht et al., 1994). The data suggest that lenograstim was beneficial for patients < 15 years of age, as well as for the general population. No difference was seen between the lenograstim and placebo groups in the frequency of infections, culture-confirmed infections, or neutropenic fever, but the durations of infections and fever were shorter in lenograstim-treated pa-tients. Use of lenograstim also shortened days of hospitalization, antibacterial use, and parenteral nutrition compared with placebo.
Sargramostim
Sargramostim is indicated to accelerate myeloid recovery after autologous bone marrow transplanta-tion in patients with non-Hodgkin’s lymphoma, acute lymphoblastic leukemia, or Hodgkin’s disease. It is also indicated in patients undergoing allogeneic transplantation from human leukocyte antigen (HLA)-matched related donors. In a double-blind, randomized, placebo-controlled study, 128 patients underwent autologous bone marrow transplantation for lymphoid cancer. Sargramostim administered by intravenous infusion over 2 hours was started within4 hours after bone marrow infusion and continued for 21 days. Sargramostim shortened the duration of neutropenia, antibiotic therapy, and hospitalization (Nemunaitis et al., 1991).
Molgramostim
Molgramostim is indicated to reduce the duration of neutropenia and its sequelae after bone marrow transplantation for non-myeloid malignancies. Molgramostim accelerated neutrophil recovery in a randomized phase III study in patients with delayed engraftment. Although molgramostim allowed recovery of neutrophil counts, some patients did not respond, and a few responded to treatment but subsequently died (Klingemann et al., 1990; Brandwein et al., 1991).
Peripheral Blood Progenitor Cell Mobilization forHarvesting and Transplantation
Stem (progenitor) cells found in the blood can be collected and concentrated for infusion after myelo-suppressive cancer chemotherapy. PBPC harvesting (or collection) is attractive because it is less invasive than bone marrow harvesting. PBPC harvesting can be performed in the outpatient setting without anesthetizing the donor; it causes less morbidity and mortality, costs less, circumvents donor problems, and is suitable for a larger number of patients.
Hematopoietic growth factors expand the popu-lation of circulating hematopoietic progenitor cells and may be used to facilitate peripheral collection, which in turn can be used to supplement and/or replace autologous bone marrow collection. Hematopoietic growth factors can either be combined with chemotherapy to enhance the mobilizing effect of the latter or used alone to induce de novo mobilization.
Filgrastim
In the United States, filgrastim is indicated to mobilize PBPCs for collection by leukapheresis in patients undergoing myelosuppressive or myeloablative ther-apy followed by transplantation. In Europe, filgrastim is indicated to mobilize PBPCs in patients undergoing myelosuppressive or myeloablative therapy followed by autologous PBPC transplantation with or without bone marrow transplantation.
Seventeen patients with non-myeloid malignan-cies who received filgrastim by continuous subcuta-neous infusion had a 58-fold increase in the numbers of granulocyte-macrophage progenitor cells (CFU-GM) in peripheral blood (Sheridan et al., 1992). Progenitor cells were collected by three leukapheresis procedures and infused after high-dose chemotherapy to augment autologous bone marrow rescue and post-transplant filgrastim therapy. The time to plateletrecovery was shorter in patients who received filgrastim-mobilized PBPCs compared with controls. A prospective randomized trial in lymphoma patients compared the effects of filgrastim-mobilized PBPCs or autologous bone marrow infused after high-dose chemotherapy. In this study, filgrastim-mobilized PBPCs significantly reduced the number of platelet transfusions and the time to platelet and neutrophil recovery. It also led to an earlier hospital discharge compared with patients receiving autologous marrow (Schmitz et al., 1996).
Sargramostim
Sargramostim is indicated to mobilize PBPCs in patients undergoing myelosuppressive or myeloabla-tive therapy followed by autologous PBPC transplan-tation. It is also indicated to further accelerate myeloid recovery after PBPC transplantation. A trial of sargra-mostim alone, filgrastim alone, or the combination in normal donors showed a greater median CD34þ cell yield with the combination or with filgrastim alone compared with sargramostim alone (Lane et al., 1995).
A prospective, randomized, open-label trial directly compared the effects of filgrastim and sargramostim used prophylactically in hematologic recovery and resource utilization after myelosuppres-sive chemotherapy (Weaver et al., 2000). One hundred and fifty-eight patients with breast cancer, malignant lymphoma, or multiple myeloma were enrolled and received myelosuppressive chemotherapy. Starting the day after the completion of chemotherapy, patients received filgrastim or sargramostim or the combination of sargramostim followed by filgrastim. Patients treated with filgrastim alone had significantly faster neutrophil recovery, lower incidence of fever, less intravenous antibiotic use, and fewer hospital admissions compared with patients treated with sargramostim alone. Patients treated with sargramos-tim followed by filgrastim had significantly faster neutrophil recovery, lower incidence of fever, less intravenous antibiotic use, and fewer hospital admis-sions compared with patients treated with sargramos-tim alone. Patients treated with filgrastim alone did not have significant differences in clinical endpoints compared with patients treated with the growth factor combination, but did have a more rapid neutrophil recovery.
Molgramostim
Molgramostim has been studied in combination and in sequence with rhG-CSF for mobilization of PBPCs (Winter et al., 1996). The combination of the two growth factors resulted in dramatic and sustained increases in peripheral blood GM-CFUs. In patients receiving rhG-CSF with molgramostim, PBPC cell content increased nearly 80-fold.
Ancestim
A number of randomized, controlled studies, includ-ing a pivotal phase III trial, were done to evaluate the efficacy of ancestim plus filgrastim compared with filgrastim alone to mobilize PBPCs in patients who were chemotherapy naive or who had received moderate to extensive prior chemotherapy or radia-tion therapy. Patients in these studies had tumors that are often treated with high-dose chemotherapy and PBPC support (i.e., breast cancer, non-Hodgkin’s lymphoma, Hodgkin’s disease, myeloma, and ovarian cancer). The phase III trial evaluated ancestim plus filgrastim in a cytokine-only mobilization regimen (Shpall et al., 1999). The primary endpoint of this study was reduction in the number of leukaphereses. The combination of ancestim and filgrastim resulted in a statistically significant reduction in the number of leukaphereses needed to reach the CD34þ cell target compared with mobilization with filgrastim alone. A median of four leukaphereses was needed in the ancestim plus filgrastim group compared with six or more in the filgrastim-alone group.
Severe Chronic Neutropenia
Severe chronic neutropenia may be present from birth (congenital), be periodic (cyclic), or have an unknown etiology (idiopathic). The condition is manifested by decreased neutrophil counts, recurrent fever, chronic oropharyngeal inflammation, and severe infection.
Filgrastim
Filgrastim is indicated to reduce the incidence and duration of these sequelae. In a phase III study, 123 patients were randomized to receive filgrastim immediately or after a 4-months observation period (Dale et al., 1993). Filgrastim was given by subcuta-neous injection at doses of 3.45 mg/kg/day for idiopathic neutropenia, 5.75 mg/kg/day for cyclic neutropenia, and 11.50 mg/kg twice daily for conge-nital neutropenia. The dose was adjusted to maintain the median monthly absolute neutrophil count be-tween 1.5 and 10 109/L. Hematologic responses were evident within a few days. Ninety percent of patients achieved complete responses: improved bone marrow morphology and a lower incidence and duration of infection-related events.
Related Topics