Chapter: Essential Microbiology: Microorganisms in the Environment
The sulphur cycle - Microorganisms in the Environment
The sulphur
cycle
Sulphur is found in living organisms in the form of
compounds such as amino acids, coenzymes and vitamins. It can be utilised by
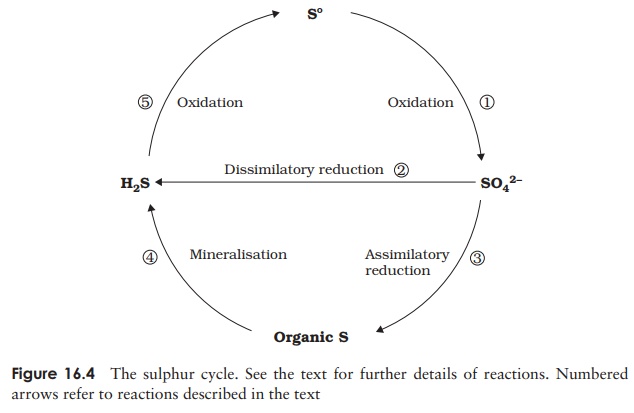
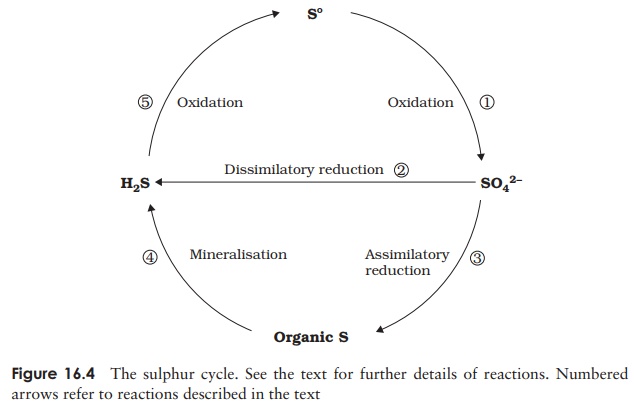
different types of organisms in several forms; Figure 16.4 shows the principal
components of the sulphur cycle.
In its elemental form, sulphur is unavailable to most
organisms; however, certain bacteria such as Acidithiobacillus are able to oxidise it to sulphate (1), a form that can be utilised by a
much broader range of organisms:
2S + 3O2+ 2H2O−−−−−−−→H2SO4
Powdered sulphur is often added to alkaline soils in
order to encourage this reaction and thereby reduce the pH.
Sulphate-reducing bacteria convert the sulphate to
hydrogen sulphide gas (2) using
either an organic compound or hydrogen gas as electron donor:
8H++ SO42−−−−−−−−→H2S + 2H2O + 2OH−
These bacteria are obligate anaerobes, and the
process is termed dissimilatory
sulphate reduction.
Plants are also able to utilise sulphate,
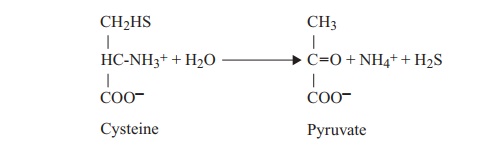
incorporating it into cellular constituents such as the amino acids methionine
and cysteine (3) (assimilatory sulphate reduction).
When the plants die, these compounds are broken down,
again with the release of hydrogen sulphide (4) (see mineralisation, above).
Green and purple photosynthetic bacteria and some
chemoautotrophs use hydrogen sulphide as an electron donor in the reduction of
carbon dioxide, producing elemental sulphur and thus completing the cycle (5):
H2S + CO2−−−−−−−→(CH2O)n
+ S0
Related Topics