Chapter: Essential Microbiology: Microorganisms in the Environment
The nitrogen cycle - Microorganisms in the Environment
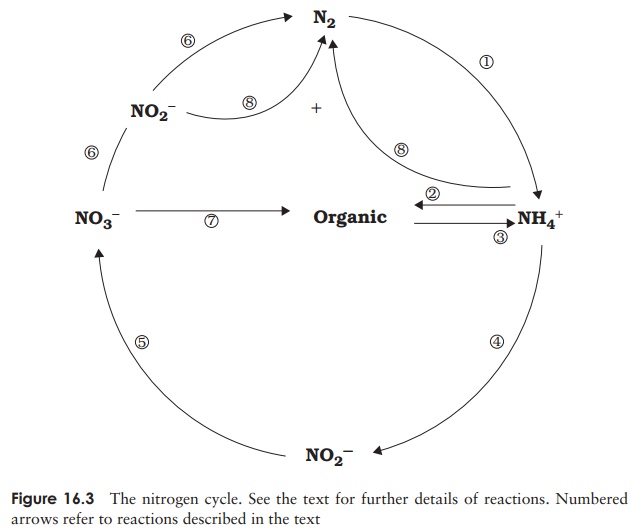
The nitrogen
cycle
Nitrogen is essential to all living things as a
component of proteins and nucleic acids. Although elemental nitrogen makes up
three quarters of the Earth’s atmosphere, only a handful of life forms are able
to utilise it for metabolic purposes. These are termed nitrogen-fixing
bacteria, and incorporate the nitrogen into ammonia (Figure 16.3, reaction 1):
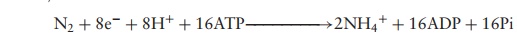
The nitrogenase enzyme complex responsible for the
reaction is very sensitive to oxygen, and is thought to have evolved early in
the Earth’s history, when the atmosphere was still largely oxygen-free. Many
nitrogen-fixing bacteria are anaerobes; those that are not have devised ways of
keeping the cell interior anoxic. Azotobacter
species, for example, utilise oxygen at a high rate, so that it never
accumulates in the cell, inactivating the nitrogenase. Many cyanophytes
(blue-greens) carry out nitrogen fixation in thick-walled heterocysts which
help maintain anoxic conditions.
Some nitrogen-fixing bacteria such as Rhizobium infect the roots of leguminous
plants such as peas, beans and clover, where they form nodules and form a
mutually beneficial association.
Ammonia produced by nitrogen fixation is assimilated
as amino acids, which can then form proteins and feed into pathways of
nucleotide synthesis (2). Organic nitrogen in the form of dead plant and animal
material plus excrement re-enters the environment, where it undergoes
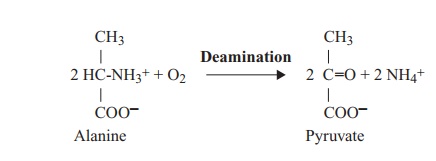
mineralisation (3) at the hands of a range of microorganisms, involving the
deamination of amino acids to their corresponding organic acid. This process of
mineralisation may occur aerobically or anaerobically, in a wide range of
microorganisms, e.g.:
The process of nitrification,
by which ammonia is oxidised stepwise firstly to nitrite and then to nitrate,
involves two different groups of bacteria (4,
5).
NH4+−→ NO2−
NO2−−→ NO3−
The nitrate thus formed may suffer a number of fates. It may act
as an electron acceptor in anaerobic respiration, becoming reduced to nitrogen via
a series of intermedi-ates including nitrite (6). This process of denitrification
occurs in anaerobic conditions such as waterlogged soils. Alternatively, it can
be reduced once again to ammonia and thence converted to organic nitrogen (7).
A final pathway of nitrogen cycling has only been
dis-covered in recent years. It is known as anammox
(anaer-obic ammonia oxidation), and is carried out by members of a group of
Gram-negative bacteria called the Planc-tomycetes. The reaction, which can be
represented thus:
NH4+ + NO2− = N2
+ 2H2O (8)
has considerable potential in the removal of nitrogen
from wastewater.
Related Topics