Chapter: Basic & Clinical Pharmacology : Pharmacokinetics & Pharmacodynamics: Rational Dosing & the Time Course of Drug Action
The Target Concentration Approach to Designing a Rational Dosage Regimen
THE TARGET CONCENTRATION APPROACH
TO DESIGNING A RATIONAL DOSAGE REGIMEN
A
rational dosage regimen is based on the assumption that there is a target concentration that will produce
the desired therapeutic effect. By considering the pharmacokinetic factors that
determine the dose-concentration relationship, it is possible to individualize
the dose regimen to achieve the target concentration. The effective
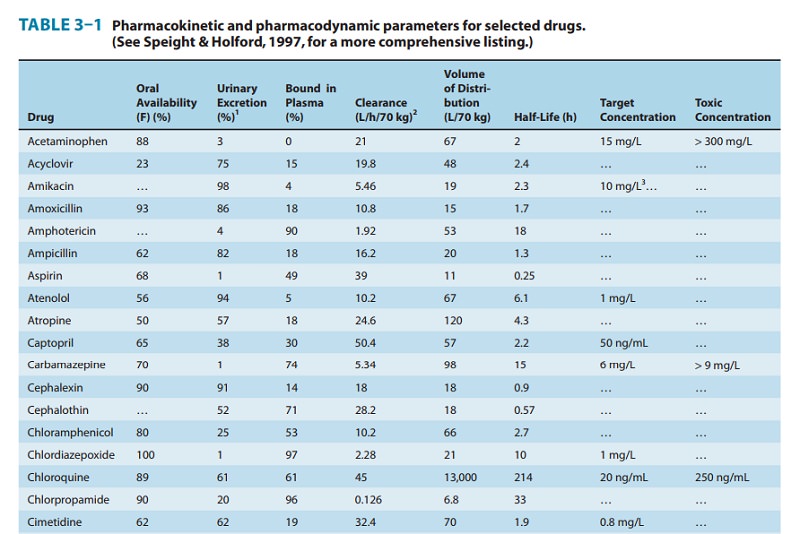
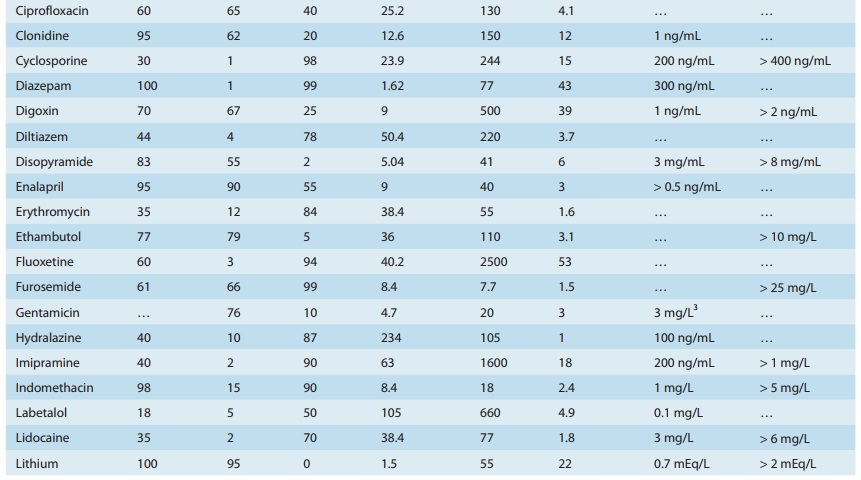
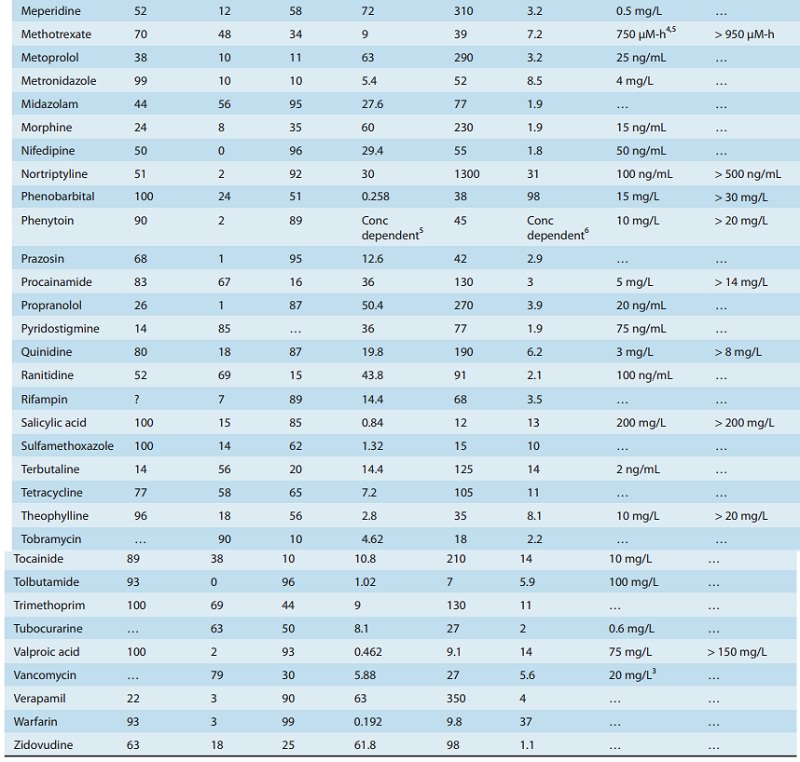
concentration ranges shown in Table 3–1 are a guide to the con-centrations
measured when patients are being effectively treated. The initial target
concentration should usually be chosen from the lower end of this range. In
some cases, the target concentration will also depend on the specific
therapeutic objective—eg, the control of atrial fibrillation by digoxin often
requires a target concentration of 2 ng/mL, while heart failure is usually
adequately managed with a target concentration of 1 ng/mL.
Maintenance Dose
In
most clinical situations, drugs are administered in such a way as to maintain a
steady state of drug in the body, ie, just enough drug is given in each dose to
replace the drug eliminated since the preceding dose. Thus, calculation of the
appropriate maintenance dose is a primary goal. Clearance is the most important
pharma-cokinetic term to be considered in defining a rational steady-state drug
dosage regimen. At steady state, the dosing rate (“rate in”) must equal the
rate of elimination (“rate out”). Substitution of the target concentration (TC)
for concentration (C) in equation (4) predicts the maintenance dosing rate:

Thus,
if the desired target concentration is known, the clearance in that patient
will determine the dosing rate. If the drug is given by a route that has a
bioavailability less than 100%, then the dosing rate predicted by equation (9)
must be modified. For oral dosing:

If
intermittent doses are given, the maintenance dose is calcu-lated from:

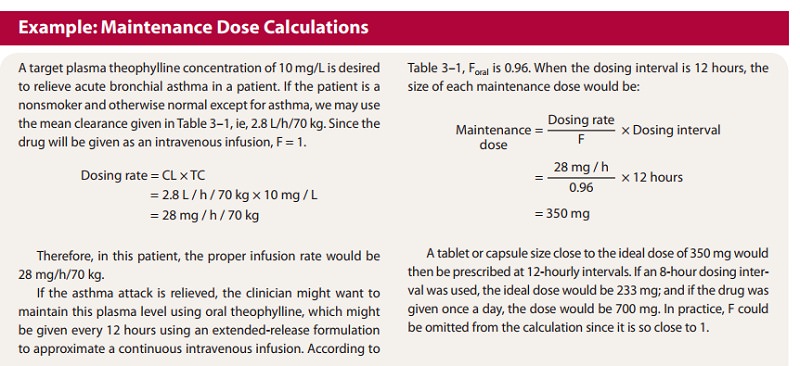
(See
Box: Example: Maintenance Dose Calculations.)
Note
that the steady-state concentration achieved by con-tinuous infusion or the
average concentration following inter-mittent dosing depends only on clearance.
The volume of distribution and the half-life need not be known in order to
determine the average plasma concentration expected from a given dosing rate or
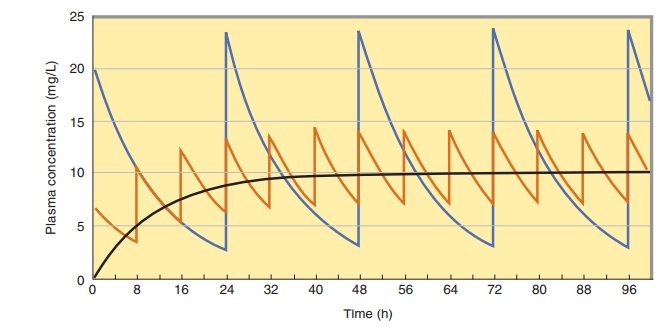
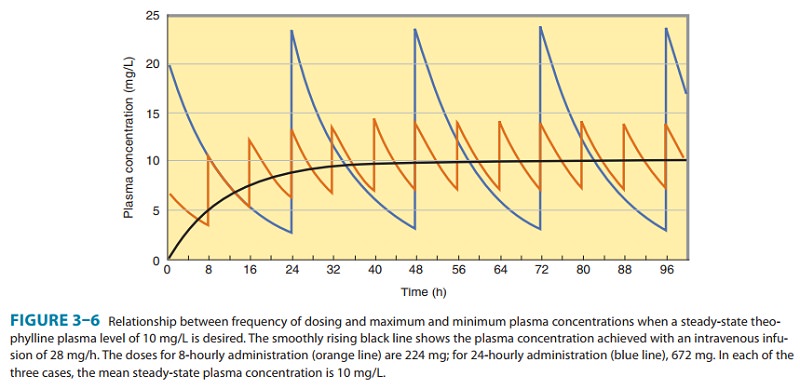
to predict the dosing rate for a desired tar-get concentration. Figure 3–6 shows
that at different dosing intervals, the concentration-time curves will have
different maximum and minimum values even though the average level will always
be 10 mg/L.
Estimates
of dosing rate and average steady-state concentra-tions, which may be calculated
using clearance, are independent of any specific pharmacokinetic model. In
contrast, the determi-nation of maximum and minimum steady-state concentrations
requires further assumptions about the pharmacokinetic model. The accumulation
factor (equation [7]) assumes that the drug fol-lows a one-compartment body
model (Figure 3–2B), and the peak concentration prediction assumes that the
absorption rate is much faster than the elimination rate. For the calculation
of estimated maximum and minimum concentrations in a clinical situation, these
assumptions are usually reasonable.
Loading Dose
When
the time to reach steady state is appreciable, as it is for drugs with long
half-lives, it may be desirable to administer a loading dose that promptly
raises the concentration of drug in plasma to the target concentration. In
theory, only the amount of the load-ing dose need be computed—not the rate of
its administration— and, to a first approximation, this is so. The volume of
distribution is the proportionality factor that relates the total amount of
drug in the body to the concentration; if a loading dose is to achieve the
target concentration, then from equation (1):

For
the theophylline example given in the Box, Example: Maintenance Dose
Calculations, the loading dose would be 350 mg (35 L × 10 mg/L) for a 70-kg person. For most drugs,
the loading dose can be given as a single dose by the chosen route of
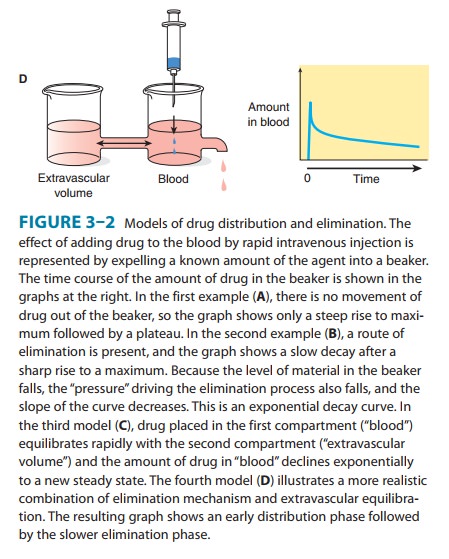
administration. Up to this point, we have ignored the fact that some drugs
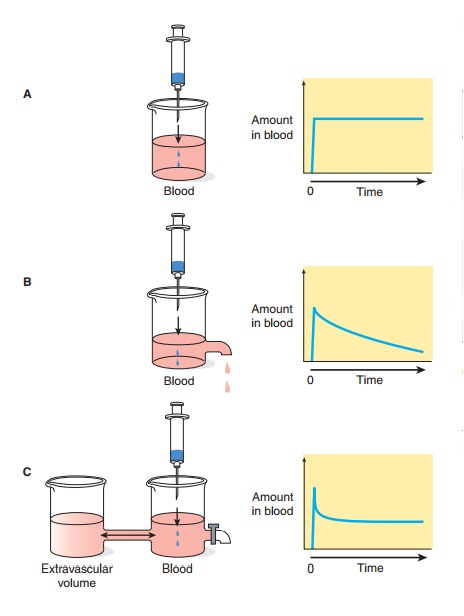
follow more complex multicompartment pharmacokinetics, eg, the distribution
process illustrated by the two-compartment model in Figure 3–2. This is
justified in the great majority of cases. However, in some cases the distribution
phase may not be ignored, particularly in connection with the calculation of
load-ing doses. If the rate of absorption is rapid relative to distribution
(this is always true for rapid intravenous administration), the concentration
of drug in plasma that results from an appropriate loading dose—calculated
using the apparent volume of distribu-tion—can initially be considerably higher
than desired. Severe toxicity may occur, albeit transiently. This may be
particularly important, eg, in the administration of antiarrhythmic drugs such
as lidocaine, where an almost immediate toxic response may occur. Thus, while
the estimation of the amount of a
loading dose may be quite correct, the rate
of administration can sometimes be crucial in preventing excessive drug
concentrations, and slow administration of an intravenous drug (over minutes
rather than seconds) is almost always prudent practice.
When
intermittent doses are given, the loading dose calculated from equation (12)
will only reach the average steady-state concentration and will not match the
peak steady-state concentration (Figure 3–6). To match the peak steady-state
con-centration, the loading dose can be calculated from equation (13):

Related Topics