Chapter: Basic & Clinical Pharmacology : Adrenocorticosteroids And Adrenocortical Antagonists
The Naturally Occurring Glucocorticoids; Cortisol (Hydrocortisone)
THE NATURALLY OCCURRING
GLUCOCORTICOIDS; CORTISOL (HYDROCORTISONE)
Pharmacokinetics
Cortisol (also called
hydrocortisone, compound F) exerts a wide range of physiologic effects,
including regulation of intermediary metabolism, cardiovascular function,
growth, and immunity. Its synthesis and secretion are tightly regulated by the
central nervous system, which is very sensitive to negative feedback by the
circulat-ing cortisol and exogenous (synthetic) glucocorticoids. Cortisol is
synthesized from cholesterol (as shown in Figure 39–1).
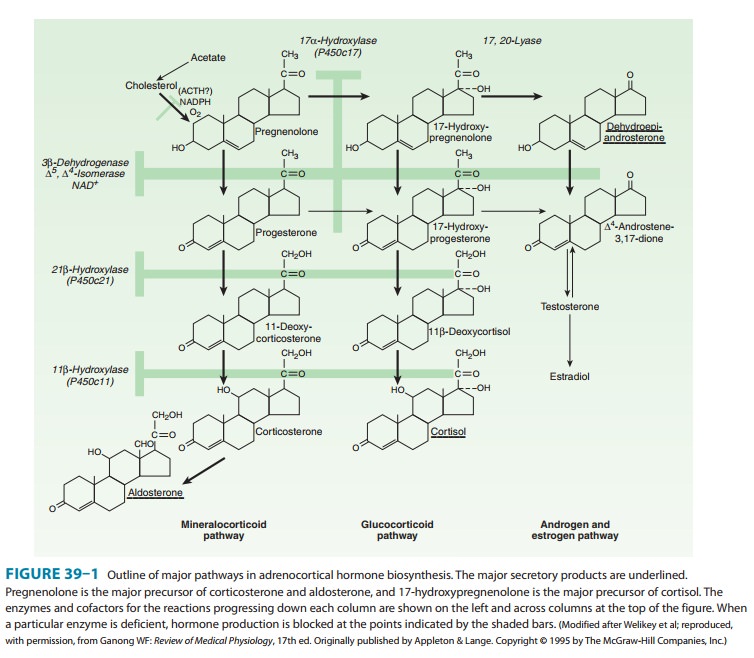
In
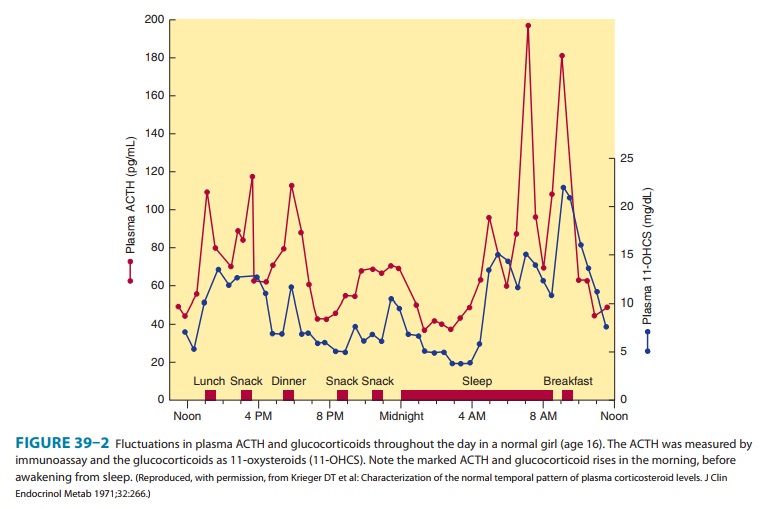
the normal adult, in the absence of stress, 10–20 mg of cortisol is secreted
daily. The rate of secretion follows a circadian rhythm governed by pulses of
ACTH that peak in the early morning hours and after meals, especially after
lunch (Figure 39–2). In plasma, cortisol is bound to circulating proteins.
Corticosteroid-binding globulin (CBG), an α2
globulin synthesized by the liver, binds about 90% of the circulating hormone
under normal cir-cumstances. The remainder is free (about 5–10%) or loosely
bound to albumin (about 5%) and is available to exert its effect on target
cells. When plasma cortisol levels exceed 20–30 mcg/dL, CBG is saturated, and
the concentration of free cortisol rises rapidly. CBG is increased in pregnancy
and with estrogen admin-istration and in hyperthyroidism. It is decreased by
hypothyroid-ism, genetic defects in synthesis, and protein deficiency states.
Albumin has a large capacity but low affinity for cortisol, and for practical
purposes albumin-bound cortisol should be considered free. Synthetic
corticosteroids such as dexamethasone are largely bound to albumin rather than
CBG.
The
half-life of cortisol in the circulation is normally about 60–90 minutes; it
may be increased when hydrocortisone (the pharmaceutical preparation of
cortisol) is administered in large
Only 1% of
cortisol is excreted unchanged in the urine as free cortisol; about 20% of
cortisol is converted to cortisone by 11-hydroxysteroid dehydrogenase in the
kidney and other tissues with mineralocorticoid receptors before reaching the liver. Most cortisol is
metabolized in the liver. About one third of the cortisol produced daily is
excreted in the urine as dihydroxy ketone metabolites and is measured as
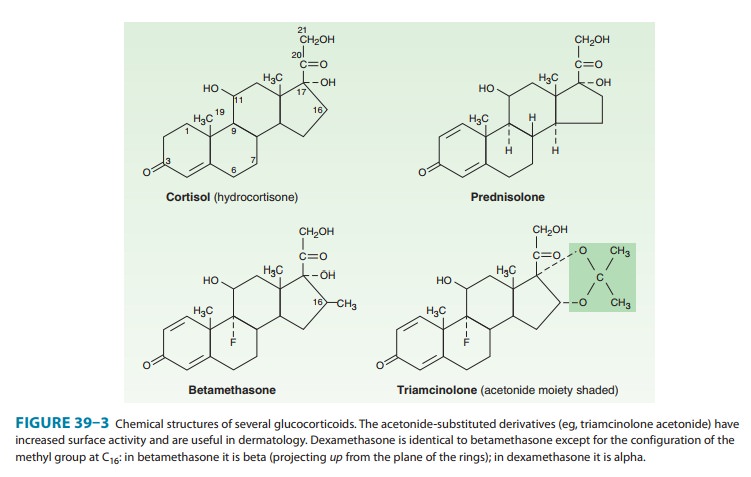
17-hydroxysteroids (see Figure 39–3 for carbon numbering). Many cortisol
metabolites are conjugated with glucuronic acid or sulfate at the C3
and C21 hydroxyls, respectively, in the
liver; they are then excreted in the urine.
In
some species (eg, the rat), corticosterone is the major gluco-corticoid. It is
less firmly bound to protein and therefore metabo-lized more rapidly. The
pathways of its degradation are similar to those of cortisol.
Pharmacodynamics
A. Mechanism of Action
Most of the known
effects of the glucocorticoids are mediated by widely distributed
glucocorticoid receptors. These proteins are members of the superfamily of
nuclear receptors, which includes steroid, sterol (vitamin D), thyroid,
retinoic acid, and many other receptors with unknown or nonexistent ligands
(orphan recep-tors). All these receptors interact with the promoters of—and
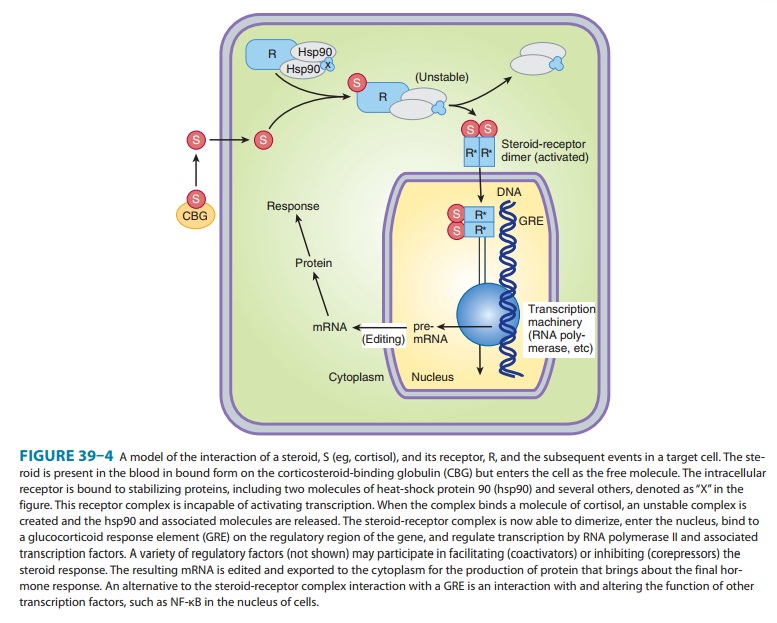
regulate the transcription of—target genes (Figure 39–4). In theabsence of the
hormonal ligand, glucocorticoid receptors are pri-marily cytoplasmic, in
oligomeric complexes with heat-shock proteins (hsp). The most important of
these are two molecules of hsp90, although other proteins are certainly
involved. Free hormone from the plasma and interstitial fluid enters the cell
and binds to the receptor, inducing conformational changes that allow it to
dissociate from the heat shock proteins. The ligand-bound receptor complex then
is actively transported into the nucleus, where it interacts with DNA and
nuclear proteins. As a homodi-mer, it binds to glucocorticoid receptor elements (GREs) in the promoters of responsive
genes. The GRE is composed of two palindromic sequences that bind to the
hormone receptor dimer.
In addition to binding
to GREs, the ligand-bound receptor also forms complexes with and influences the
function of other tran-scription factors, such as AP1 and NF-κB, which act on
non-GRE-containing promoters, to contribute to the regulation of transcription
of their responsive genes. These transcription factors have broad actions on
the regulation of growth factors, proinflammatory cytok-ines, etc, and to a
great extent mediate the anti-growth, anti-inflammatory, and immunosuppressive
effects of glucocorticoids.
Two genes for the corticoid receptor have been identified: one encoding the classic glucocorticoid receptor (GR) and the other encoding the mineralocorticoid receptor (MR). Alternative splicing of human glucocorticoid receptor pre-mRNA generates two highly homologous isoforms, termed hGR alpha and hGR beta. Human GR alpha is the classic ligand-activated glucocorticoid receptor which, in the hormone-bound state, modulates the expression of glucocorticoid-responsive genes.
In contrast, hGR beta does not bind
glucocorticoids and is transcriptionally inactive. However, hGR beta is able to
inhibit the effects of hormone-activated hGR alpha on glucocorticoid-responsive
genes, playing the role of a physiologically relevant endogenous inhibitor of
glucocorticoid action. It was recently shown that the two hGR alternative
tran-scripts have eight distinct translation initiation sites; ie, in a human
cell there may be up to 16 GRα and GRβ isoforms, which may form up to 256
homodimers and heterodimers with different tran-scriptional and possibly
non-transcriptional activities. This variabil-ity suggests that this important
class of steroid receptors has complex stochastic activities.
The
prototype glucocorticoid receptor isoform is composed of about 800 amino acids
and can be divided into three functional domains (see Figure 2–6). The
glucocorticoid-binding domain is located at the carboxyl terminal of the
molecule. The DNA-binding domain is located in the middle of the protein and
con-tains nine cysteine residues. This region folds into a “two-finger”
structure stabilized by zinc ions connected to cysteines to form two
tetrahedrons. This part of the molecule binds to the GREs that regulate
glucocorticoid action on glucocorticoid-regulated genes. The zinc fingers
represent the basic structure by which the DNA-binding domain recognizes
specific nucleic acid sequences. The amino-terminal domain is involved in the
transactivation activity of the receptor and increases its specificity.
The
interaction of glucocorticoid receptors with GREs or other transcription
factors is facilitated or inhibited by several families of proteins called
steroid receptor coregulators,
divided into coactivators and corepressors. The coregulators do this
by serving as bridges between the receptors and other nuclear proteins and by
expressing enzymatic activities such as histone acetylase or deacetylase, which
alter the conformation of nucleosomes and the transcribability of genes.
Between
10% and 20% of expressed genes in a cell are regu-lated by glucocorticoids. The
number and affinity of receptors for the hormone, the complement of
transcription factors and coregu-lators, and post-transcription events
determine the relative speci-ficity of these hormones’ actions in various
cells. The effects of glucocorticoids are mainly due to proteins synthesized
from mRNA transcribed from their target genes.
Some
of the effects of glucocorticoids can be attributed to their binding to
mineralocorticoid receptors (MRs). Indeed, MRs bind aldosterone and cortisol
with similar affinity. A mineralocorticoid effect of cortisol is avoided in
some tissues by expression of 11β-hydroxysteroid dehydrogenase type
2, the enzyme responsible for biotransformation to its 11-keto derivative
(cortisone), which has minimal affinity for aldosterone receptors.
Recently, the GR was found to interact with CLOCK/BMAL-1, a transcription factor dimer expressed in all tissues and generating the circadian rhythm of cortisol secretion at the suprachiasmatic nucleus of the hypothalamus. CLOCK is an acetyltransferase that acetylates the hinge region of the GR, neutralizing its transcrip-tional activity and thus rendering target tissues resistant to gluco-corticoids. Interestingly, the glucocorticoid target tissue sensitivity rhythm generated is in reverse phase to that of circulating cortisol concentrations, explaining the increased sensitivity of the organism to evening administration of glucocorticoids.
Prompt
effects such as initial feedback suppression of pituitary ACTH occur in minutes
and are too rapid to be explained on the basis of gene transcription and
protein synthesis. It is not known how these effects are mediated. Among the
proposed mechanisms are direct effects on cell membrane receptors for the
hormone or nonge-nomic effects of the classic hormone-bound glucocorticoid
receptor. The putative membrane receptors might be entirely different from the
known intracellular receptors. Recently, all steroid receptors (except the MRs)
were shown to have palmitoylation motifs that allow enzymatic addition of
palmitate and increased localization of the receptors in the vicinity of plasma
membranes. Such receptors are available for direct interactions with and
effects on various membrane-associated or cytoplasmic proteins without the need
for entry into the nucleus and induction of transcriptional actions.
B. Physiologic Effects
The
glucocorticoids have widespread effects because they influ-ence the function of
most cells in the body. The major metabolic consequences of glucocorticoid
secretion or administration are due to direct actions of these hormones in the
cell. However, some important effects are the result of homeostatic responses
by insulin and glucagon. Although many of the effects of glucocorticoids are
dose-related and become magnified when large amounts are administered for
therapeutic purposes, there are also other effects— called permissive effects—without which many normal functions become
deficient. For example, the response of vascular and bron-chial smooth muscle
to catecholamines is diminished in the absence of cortisol and restored by
physiologic amounts of this glucocorticoid. Similarly, the lipolytic responses
of fat cells to catecholamines, ACTH, and growth hormone are attenuated in the
absence of glucocorticoids.
C. Metabolic Effects
The glucocorticoids
have important dose-related effects on carbo-hydrate, protein, and fat
metabolism. The same effects are respon-sible for some of the serious adverse
effects associated with their use in therapeutic doses. Glucocorticoids
stimulate and are required for gluconeogenesis and glycogen synthesis in the
fasting state.
They stimulate
phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, and glycogen synthase
and the release of amino acids in the course of muscle catabolism.
Glucocorticoids
increase serum glucose levels and thus stimu-late insulin release and inhibit
the uptake of glucose by muscle cells, while they stimulate hormone sensitive
lipase and thus lipolysis. The increased insulin secretion stimulates
lipogenesis and to a lesser degree inhibits lipolysis, leading to a net
increase in fat deposition combined with increased release of fatty acids and
glycerol into the circulation.
The net results of
these actions are most apparent in the fasting state, when the supply of
glucose from gluconeogenesis, the release of amino acids from muscle
catabolism, the inhibition of periph-eral glucose uptake, and the stimulation
of lipolysis all contribute to maintenance of an adequate glucose supply to the
brain.
D. Catabolic and Antianabolic Effects
Although
glucocorticoids stimulate RNA and protein synthesis in the liver, they have
catabolic and antianabolic effects in lym-phoid and connective tissue, muscle,
peripheral fat, and skin. Supraphysiologic amounts of glucocorticoids lead to
decreased muscle mass and weakness and thinning of the skin. Catabolic and
antianabolic effects on bone are the cause of osteoporosis in Cushing’s
syndrome and impose a major limitation in the long-term therapeutic use of
glucocorticoids. In children, glucocorti-coids reduce growth. This effect may
be partially prevented by administration of growth hormone in high doses, but
this use of growth hormone is not recommended.
E. Anti-Inflammatory and Immunosuppressive Effects
Glucocorticoids
dramatically reduce the manifestations of inflam-mation. This is due to their
profound effects on the concentration, distribution, and function of peripheral
leukocytes and to their suppressive effects on the inflammatory cytokines and
chemokines and on other mediators of inflammation. Inflammation, regardless of its
cause, is characterized by the extravasation and infiltration of leukocytes
into the affected tissue. These events are mediated by a complex series of
interactions of white cell adhesion molecules with those on endothelial cells
and are inhibited by glucocorti-coids. After a single dose of a short-acting
glucocorticoid, the concentration of neutrophils in the circulation increases
while the lymphocytes (T and B cells), monocytes, eosinophils, and baso-phils
decrease. The changes are maximal at 6 hours and are dissi-pated in 24 hours.
The increase in neutrophils is due both to the increased influx into the blood
from the bone marrow and to the decreased migration from the blood vessels,
leading to a reduction in the number of cells at the site of inflammation. The
reduction in circulating lymphocytes, monocytes, eosinophils, and basophils is
primarily the result of their movement from the vascular bed to lymphoid
tissue.
Glucocorticoids also
inhibit the functions of tissue mac-rophages and other antigen-presenting
cells. The ability of these cells to respond to antigens and mitogens is
reduced. The effect on macrophages is particularly marked and limits their
ability to phagocytose and kill microorganisms and to produce tumornecrosis factor-α, interleukin-1, metalloproteinases,
and plasmi-nogen activator. Both macrophages and lymphocytes produce less
interleukin-12 and interferon-γ, important inducers of TH1 cell activity, and cellular immunity.
In addition to their
effects on leukocyte function, glucocorti-coids influence the inflammatory
response by reducing the pros-taglandin, leukotriene, and platelet-activating
factor synthesis that results from activation of phospholipase A2. Finally,
gluco-corticoids reduce expression of cyclooxygenase-2, the inducible form of
this enzyme, in inflammatory cells, thus reducing the amount of enzyme
available to produce prostaglandins.
Glucocorticoids cause
vasoconstriction when applied directly to the skin, possibly by suppressing
mast cell degranulation. They also decrease capillary permeability by reducing
the amount of histamine released by basophils and mast cells.
The anti-inflammatory
and immunosuppressive effects of glu-cocorticoids are largely due to the
actions described above. In humans, complement activation is unaltered, but its
effects are inhibited. Antibody production can be reduced by large doses of
steroids, although it is unaffected by moderate doses (eg, 20 mg/d of
prednisone).
The anti-inflammatory
and immunosuppressive effects of these agents are widely useful therapeutically
but are also respon-sible for some of their most serious adverse effects (see
text that follows).
F. Other Effects
Glucocorticoids have
important effects on the nervous system. Adrenal insufficiency causes marked
slowing of the alpha rhythm of the electroencephalogram and is associated with
depression. Increased amounts of glucocorticoids often produce behavioral
disturbances in humans: initially insomnia and euphoria and sub-sequently
depression. Large doses of glucocorticoids may increase intracranial pressure
(pseudotumor cerebri).
Glucocorticoids given
chronically suppress the pituitary release of ACTH, growth hormone,
thyroid-stimulating hormone, and luteinizing hormone.
Large doses of
glucocorticoids have been associated with the development of peptic ulcer,
possibly by suppressing the local immune response against Helicobacter pylori. They also promote fat redistribution in the
body, with increase of visceral, facial, nuchal, and supraclavicular fat, and
they appear to antagonize the effect of vitamin D on calcium absorption. The
glucocorticoids also have important effects on the hematopoietic system. In
addi-tion to their effects on leukocytes, they increase the number of platelets
and red blood cells.
Cortisol deficiency
results in impaired renal function (particu-larly glomerular filtration),
augmented vasopressin secretion, and diminished ability to excrete a water
load.
Glucocorticoids have
important effects on the development of the fetal lungs. Indeed, the structural
and functional changes in the lungs near term, including the production of
pulmonary sur-face-active material required for air breathing (surfactant), are
stimulated by glucocorticoids.
Related Topics