Acids and Bases | Chapter 14 | 8th Science - Student Activities | 8th Science : Chapter 14 : Acids and Bases
Chapter: 8th Science : Chapter 14 : Acids and Bases
Student Activities
Activity 1
Take a clean test tube with holder and pour some dilute hydrochloric acid. Add few pieces of magnesium ribbon slowly. What do you observe? Now show a burning match stick near the mouth of the test tube. Do you hear any sound? The gas burns with a pop sound. From this it is observed that hydrogen gas is formed due to the reaction between acid and metal (Do it under the supervision of the teacher).
Copper or brass cooking vessels are coated with tin metal (eyam). If it is not coated the organic acids present in the food materialswill react with copper and make the food poisonous. The tin isolates the vessel from the action of acids and prevents food poisoning.
Answer: The gas burns with a pop sound. From this it is observed that hydrogen gas has been formed due to the reaction between acid and metal.
Activity 2
Take some lemon juice in a tumbler and add baking soda slowly. What do you see? What do you infer from this?
Answer:
Inference : When lemon juice is mixed with baking soda, the new product CO2 is formed with water and salt.
Activity 3
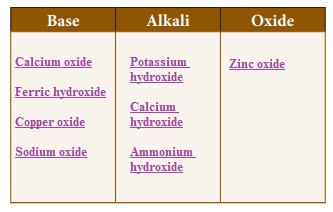
Classify the following substances.Sodium oxide, Potassium hydroxide, Calcium oxide, Copper oxide, Calcium hydroxide, Ammonium hydroxide, Ferric hydroxide, Zinc oxide
Activity 4
Take a white cloth with turmeric powder stain. Wash the cloth with washing soap. Do you observe any change in the colour? Why?
Answer: Yes, the colour changes from yellow to red, because soapy solution is a base.
Activity 5
Take a small beet root vegetable and cut it into pieces. Boil them in hot water and filter the extract. Take two test tubes. Take sodium hydroxide solution in one test tube and vinegar or lemon juice in another test tube. Add beet root extract slowly. Observe the colour change. What do you infer?
Answer:
(i) Observation : When beetroot juice is added with sodium hydroxide solution it turns into greenish yellow shows that NaOH - is a base.
(ii) When it is added with lemon juice, the colour of beetroot juice remains same shows that lemon juice is acidic.
Activity 6
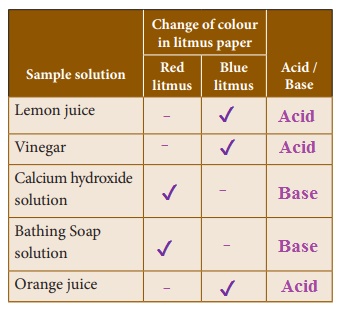
Find out the nature of the solution.
Swedish chemist Svante Arrheniu proposed a theory on acids. According to him,an acid is a substance which furnishes H+ ions or H3O+ ions in aqueous solution.

We feel hungry due to thecorrosive action of hydrochloric acid on the inner lining of the stomach. When the level of hydrochloric acidgoes higher, it causes ulcer.
Pickles remain in good condition for long time because they contain vinegar (acetic acid) or benzoic acid.

Sodium carbonate (Na2CO3) is commercially called as washing soda. Similarly sodium bicarbonate (NaHCO3) is commercially called as baking soda. Caustic soda is sodium hydroxide (NaOH) and caustic potash is potassium hydroxide (KOH).
Related Topics