Acids and Bases | Chapter 14 | 8th Science - Neutralisation Reaction | 8th Science : Chapter 14 : Acids and Bases
Chapter: 8th Science : Chapter 14 : Acids and Bases
Neutralisation Reaction
Neutralisation
Reaction
When neutrality is achieved between
two different chemical substances with different chemical properties through a
reaction then it is called neutralization in chemistry. Thus neutralization is
a chemical reaction in which an acid and a base react with each other to form
salt and water. Neutralization reaction between an acid and a base can be
written as:
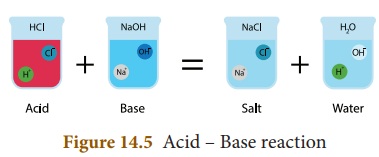
Acid
+ Base → Salt + Water
In this reaction, H+ and
Cl– ions are produced by the hydrochloric acid and Na+
and OH– ions are produced by sodium hydroxide (base). When these
ions combine together sodium chloride (NaCl) salt and water are produced.
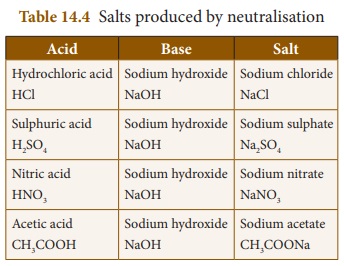
Similarly other acids also produce
their salts when they react with bases. Some of the salts produced by
neutralization reaction are given below in Table 14. 4.
Neutralisation
reactions in our daily life
Balancing acids and bases is
important for our health and for our environment. We come across various
neutralization reactions in our daily life. Let us study about the importance
of some of those reactions.
Bee bite
Whenever bees or red ants bite us
they inject an acid called formic acid into our body. This acid cause burning
sensation and pain.To suppress the pain a suitable base in the form of calcium
hydroxide (lime paste available at home) is applied so as to neutralise the
formic acid.

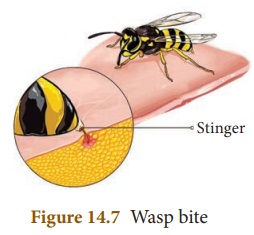
Wasp bite
When we are bitten by wasp, we feel
the burning sensation and pain. It is due to an alkaline substance injected by
the insect. To neutralise the alkalinity we use vinegar which is an acid.
Tooth decay
Generally it is advised by the
doctors that we should brush our teeth twice a day. This is because the
bacteria present in our mouth decompose the food particles stuck in the gaps
between our teeth thereby causing acid formation which leads to tooth decay. To
prevent this we have to neutralize the acid. When we brush with tooth powder or
tooth paste containing weak bases, the acid gets neutralized. So our teeth will
be strong and healthy.
Acidity
As we know, hydrochloric acid
present in our stomach helps the digestion of food material along with the
enzymes secreted by liver, gallbladder and pancreas. Sometimes due to excessive
production of hydrochloric acid in our stomach we feel burning sensation in
food pipe and in chest area. If this happens again and again ulcer will be
formed in stomach and food pipe, which further aggravates the conditions. In
order to neutralize, antacids which are nothing but weak bases like aluminum
and magnesium hydroxides are used. As a result the acidity is removed.
Agriculture
Acidic soil is not suitable for
plant growth. So farmers add lime fertilisers such as powdered lime (CaO),
limestone (CaCO3) or ashes of burnt wood to the soil to neutralise
the acidity.

Industries
Effluents from the industries
contain acids such as sulphuric acid. It is treated by adding lime to
neutralise it before it is discharged into rivers and streams. Similarly, in
power stations fossil fuels such as coal are burnt to produce electricity.
Burning fossil fuels will liberate sulphur dioxide gas as an acidic pollutant
in the air. Hence, power stations treat this acidic gas using powdered lime
(CaO) or limestone (CaCO3) to neutralise it so that air pollutant
can be prevented.

Related Topics