Chapter: Biochemistry: Transcription of the Genetic Code: The Biosynthesis of RNA
Structural Motifs in DNA-Binding Proteins
Structural Motifs in DNA-Binding
Proteins
Proteins
that bind to DNA during the course of transcription do so by the same types of
interactions that we have seen in protein structures and enzymes- hydrogen
bonding, electrostatic attractions, and hydrophobic interactions. Most proteins
that activate or inhibit transcription by RNA polymerase II have two functional
domains. One of them is the DNA-binding
domain, and the other is the transcription-activation
domain.
What are DNA-binding domains?
DNA-Binding Domains
Most
DNA-binding proteins have domains that fall into one of three categories: helix–turn–helix (HTH), zinc fingers, and basic-region leucine zipper (bZIP). These
domains interact with DNA in either the major or minor groove, with the major
groove being more common.
Helix–Turn–Helix Motifs
A common
feature seen in proteins that bind to DNA is the presence of a segment of α-helix that fits into the
major groove. The width of the major groove and the α-helix are similar, so the protein helix can
fit snugly. This is the most common motif because the standard form of DNA,
B-DNA, has the major groove of the correct size, and no alterations in its
topology are necessary. Such binding proteins are often dimers with two regions
of HTH, as shown in Figure 11.25.
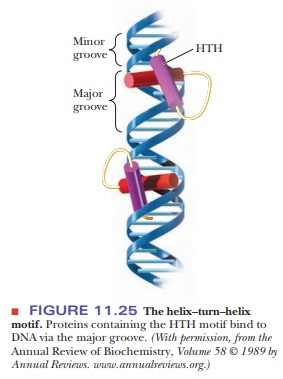
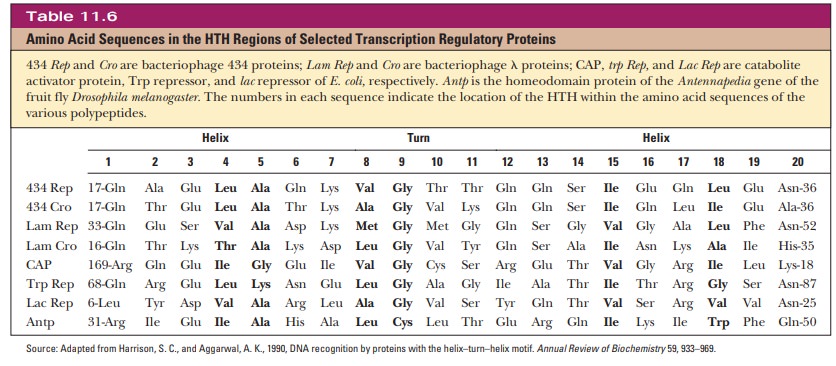
The HTH motif is a sequence of 20 amino acids that is relatively conserved in many different DNA-binding proteins. Table 11.6 shows the sequence for the HTH region of several transcription factors. The first helical region is composed of the first eight residues of the region. A sequence of three or four amino acids separates it from the second helical region. Position 9 is a glycine involved in a β-turn.
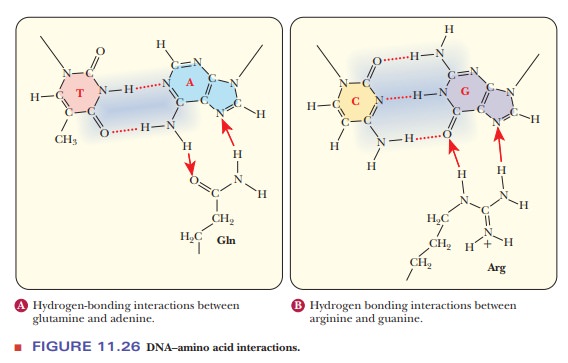
Proteins that recognize DNA with specific base sequences are more likely to bind to the major groove. The orientation of the bases in the standard base pairings puts more of the unique structure into the major groove. Figure 11.26 shows how glutamine and arginine can interact favorably with adenine and guanine, respectively. Some interactions, however, including many in the minor groove, only read the DNA indirectly. As discussed, the B form of DNA is not as constant as was once thought. Local variations in helix structure occur based on the actual sequence, especially when there are A–T rich areas. The bases undergo extensive propeller-twist. Many proteins bind to the edges of the bases that protrude into the minor groove. Studies have shown that artificial molecules that mimic the base protrusions into the minor groove are equally capable of binding to many transcription factors.
Thus,
although the base pairing is clearly important to DNA, sometimes the overall
shape of the bases is important for other reasons. A particular binding protein
might be recognizing not the part of the base that is involved in hydro-gen
bonds, but rather a part that is protruding into the grooves.
Zinc Fingers
In 1985,
it was discovered that a transcription factor of RNA polymerase III, TFIIIA,
had nine repeating structures of 30 amino acids each. Each repeat contained two
closely spaced cysteines and two closely spaced histidines 12 amino acids
later. It was also found that this factor had enough associated zinc ions to
bind to each of the repeats. This led to the discovery of the zinc-finger
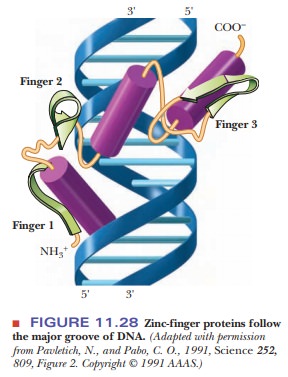
domain in DNA-binding proteins, which is represented in Figure 11.27.
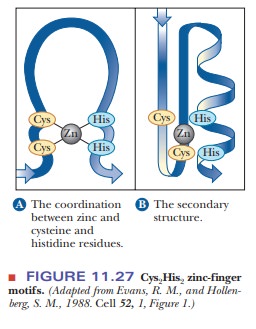
The motif gets its name from the shape adopted by the 12 amino acids that are looped out from the intersection of the two cysteines and two histidines with the zinc ion. When TFIIIA binds to DNA, the repeated zinc fingers follow the major groove around the DNA, as shown in Figure 11.28.
Basic-Region Leucine Zipper Motif
The
third major class of sequence-dependent DNA-binding proteins is called the
basic-region leucine zipper motif. Many transcription factors are known to
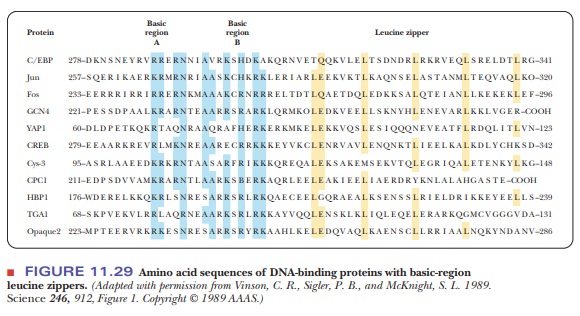
contain this motif, including CREB. Figure 11.29 shows the sequence homology of
several such transcription factors. Half of the protein is composed of the
basic region with many conserved residues of lysine, arginine, and histidine.
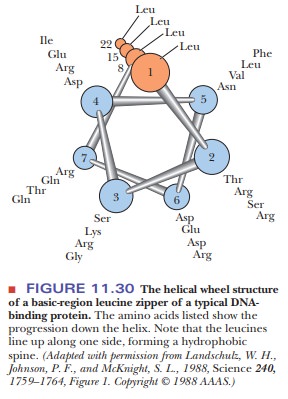
The second half contains a series of leucines every seven residues. The
significance of the spacing of the leucines is clear. It takes 3.6 amino acids
to make a turn of an α-helix. With a seven-residue spacing, the
leucines all line up on one side of an α-helix, as shown in Figure 11.30. The motif
gets the name zipper from the fact
that the line of hydrophobic residues interacts with a second analogous protein
fragment via hydrophobic bonds, interweaving themselves like a zipper.
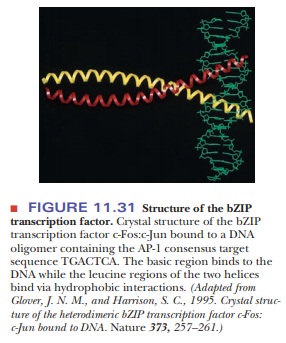
DNA-binding proteins with leucine zippers bind the DNA in the major groove via
the strong electrostatic interactions between the basic region and the sugar
phosphates. Protein dimers form, and the leucine half interacts with the other
subunit, while the basic part interacts with the DNA, as shown in Figure 11.31.
What are transcription-activation domains?
Transcription-Activation Domains
The three motifs mentioned above are involved in the binding of transcription factors to DNA. Not all transcription factors bind directly to DNA, however. Some bind to other transcription factors and never contact the DNA. An example is CBP, which bridges CREB and the RNA polymerase II transcription-initiation complex. The motifs whereby transcription factors recognize other proteins can be broken down into three categories:
·
Acidic
domains are regions rich in acidic amino acids. Gal4 is a transcriptionfactor in yeast that activates the genes
for metabolizing galactose. It has a domain of 49 amino acids, 11 of which are
acidic.
·
Glutamine-rich
domains are seen in several transcription factors. Sp1 is anupstream transcription factor that activates
transcription in the presence of an additional promoter element called a GC box. It has two glutamine-rich
domains, one of which contains 39 glutamines in 143 amino acids. CREB and
CREM also have this domain.
·
A proline-rich
domain is seen in the activator CTF-1.
It has a domain of 84 amino acids, 19 of which are prolines. CTF-1 is a member
of a class of transcription factors that bind to an extended promoter element
called a CCAAT box. The N-terminal domain has been shown to regulate
transcrip-tion of certain genes. The C-terminal end is a transcription
regulator and is known to bind to histone proteins via the proline repeats. An
active area of study is how transcription is linked to the acetylation of
histones. The coacti-vator CBP, which was discussed in the previous section, is
also a histone acetyl transferase.
Despite
the seemingly overwhelming complexity of transcription factors, their
elucidation has been made more manageable by the similarities in the motifs
described in this section. For example, if a new protein is discovered or a new
DNA sequence is elucidated, evidence of its role as a transcription factor can
be determined by locating the DNA-binding protein motifs discussed in this
section.
Summary
Proteins such as transcription factors that bind to DNA often have
recog-nizable structural motifs.
Common motifs are the helix–turn–helix, zinc fingers, and
basic-region leucine zippers.
The fact that many transcription factors and
other DNA-binding proteins have such motifs has aided in the identification of
these proteins when they are initially discovered.
In addition to binding DNA, many proteins involved in transcription
bind to other proteins.
Many
protein–protein binding domains also have identifiable motifs, such as acidic
domains, glutamine-rich domains, and proline-rich domains.
Related Topics