Chapter: Essential Clinical Immunology: Immunological Aspects of Cardiac Disease
Streptococcal Vaccine Candidate
Streptococcal Vaccine Candidate
As early as the 1930s, researchers were pursuing
the study of streptococcal vaccinations, with the injection of whole-killed
group A streptococci and cell walls thereof culminating in injections of
partially purified M protein extracts in the 1970s. However, all interest,
especially by the pharmaceutical companies, ceased at that point because the
U.S. Food and Drug Administration (FDA) proclaimed that one could work on
streptococcal vac-cines as long as no streptococcal component was used! The
FDA was afraid that induction of antibodies cross-reactive with human tissues,
especially cardiac tis-sues, could be detrimental to the vaccinee. However, it
was soon apparent that many individuals had antibodies cross-reactive with a
variety of human tissues and were perfectly normal. In the past decade, the
restriction was removed on the condition that toxicity studies in animals did
not reveal any deletion effects in the animals when injected with a
streptococcal vaccine candidate.
This ushered in the search for an effec-tive, safe,
and inexpensive group A streptococcal vaccine, and there are now at least four
prominent candidates with more in the ‚Äúpipeline.‚ÄĚ This area has recently been
reviewed in New Generation Vaccines
and will only briefly be sum-marized here.
1.
Perhaps
the most advanced candidate is by Dale and colleagues (1996), which is synthetic
peptide sequences of a vari-ety of M protein types taken from the variable
region of the M protein and hooked together by linkers. This has induced
protective immunity in ani-mals to a number of different M protein types and is
safe for use in humans. Clinical trial of its efficacy to prevent streptococcal
infections is currently under way.
2.
The use
of the C-repeat constant region of the M protein advanced by Fischetti (1989)
produces protection against oral
colonization of the throat by group A streptococci.
4.
The
streptococcal group A carbohydrate (CHO) as proposed by Zabriskie and
colleagues has been purified and used as an immunogen to protect against
streptococcal infections. This vaccine candidate promotes phagocytosis of group
A streptococci of several different M types, will protect against infection
using passive and active immunization studies, and also protects against oral
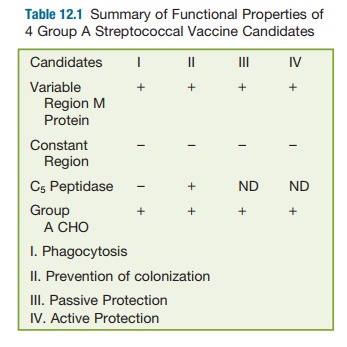
colonization. Table 12.1 summarizes all the studies on these four candidates
and it can be seen that the variable region of the M protein and the CHO
molecule offer the most convincing evidence for a protective vaccine against
group A streptococcal infections.
Related Topics