Chapter: Essential Clinical Immunology: Immunological Aspects of Cardiac Disease
Etiologic Considerations of Rheumatic Fever
Etiologic Considerations
A large body of both immunological and
epidemiological evidence has implicated the group A streptococcus in the
induction of the disease process. Yet, the precise pathological mechanisms
involved in the process remain obscure. At least three main theories have been
proposed.
The first theory is concerned with the question of
whether persistence of the organism is important. Despite several controversial
reports, no investigators have been able to consistently and reproducibly
demonstrate live organisms in rheumatic fever joints, cardiac tissues, or
valves.
The second theory focuses on whether deposition of
toxic products is required. Although an attractive hypothesis, little or no
experimental evidence has been obtained to support this concept. Renewed
interest in extracellular toxins has recently emerged with the observation that
certain strepto-coccal pyrogenic exotoxins (A and C) may act as superantigens.
These antigens may stimulate large numbers of T cells through their unique
bridging interaction with T-cell receptors of specific Vβ types and class II MHC molecules. This interaction
is clearly distinct from conventional antigen presentation in the context of
MHC. Once activated, these cells elaborate tumor necro-sis factor, gamma
interferon, and a number of interleukin moieties, thereby contribut-ing to the
initiation of pathological damage. Furthermore, it has been suggested that in
certain disease states such as rheumatoid arthritis, autoreactive cells of
specific Vβ lineage may “home” to the target
organ. Although this is an attractive hypothesis, no data concerning the role
of these superanti-gens in ARF have been forthcoming.
Perhaps the best evidence to date favors the theory
of an abnormal host immune response (both humoral and cellular) in the
genetically susceptible individual to those streptococcal antigens
cross-reactive with mammalian tissues. The evidence support-ing this theory can
be divided into three broad categories:
1.
Employing
a wide variety of methods, numerous investigators have docu-mented the presence
of heart-reactive antibodies (see Figure 12.3) in ARF sera. The prevalence of
these antibodies has varied from a low of 33 percent to a high of 85 percent in
various series. Although these antibodies are seen in other individuals
(notably those with uncomplicated streptococcal infections that do not go on to
rheumatic fever and patients with poststreptococcal glo-merulonephritis), the
titers are always lower than that seen in rheumatic fever and decrease with
time during the con-valescent period (Figure 12.4).
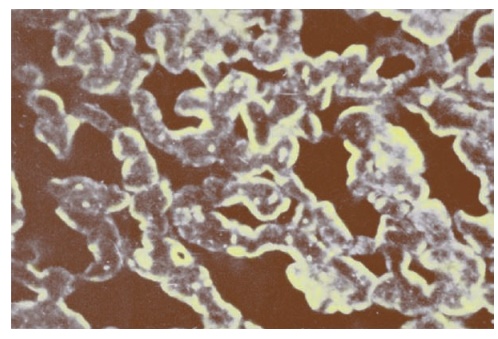
Figure 12.3 Photomicrograph of immunofluorescent staining of heart sections with a serum from an acute rheumatic fever patient followed by a fluorescein-tagged antihuman IgG goat serum. Note the intense sarcolemmal staining pattern of the serum. Identical results were obtained with rabbit sera immunized with Group A streptococcal cell walls but not Group D walls.
In terms of diagnosis and progno-sis, it is
important to observe that these heart-reactive antibody titers decline over
time. By the end of three years, these titers are essentially undetect-able in
patients who had only a single attack (see Figure 12.4) This pattern is
consistent with the well-known clinical observation that recurrences of
rheu-matic fever most often occur within the first two to three years after the
initial attack and become rarer five years after an initial episode.
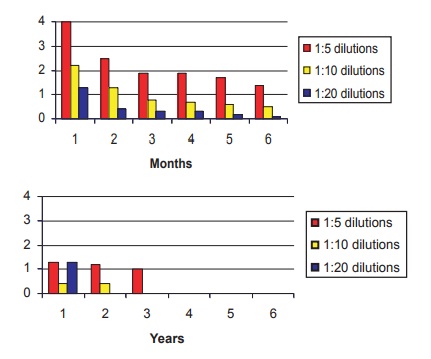
Figure 12.4 Serial heart-reactive antibody titers in forty patients with documented acute rheumatic fever. Note the slow decline of these titers over the first two years after the initial episode and the absence of these antibodies five years after the initial attack.
As illustrated in Figure 12.5, this pat-tern of titers also has prognostic value. During the two- to five-year period after the initial attack, patient M.P.’s heart-reactive titers dropped to undetectable levels. However, with a known break in prophylaxis starting in year 6, at least two streptococcal infections occurred,
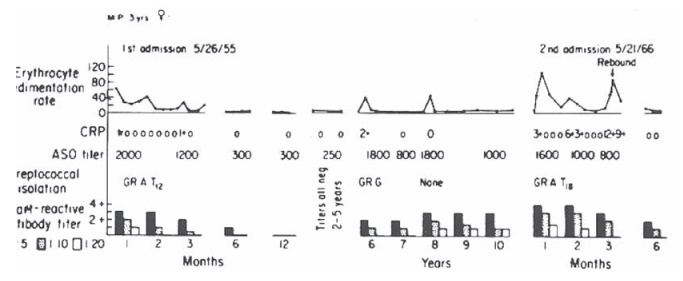
Figure 12.5 Heart-reactive antibody titers and laboratory data obtained from a patient with rheumatic fever who had two well-documented acute attacks eleven years apart. Note absence of the heart-reactive antibody during years 2 to 5 and its reappearance during years 6 to 10 after evidence of two intercurrent streptococcal infections secondary to breaks in penicillin prophylaxis (see ASO titers). High titers of heart-reactive antibody appeared with the second attack. CRP, C reactive protein. Reprinted from Kelley’s Textbook of Rheumatology, chapter 103, copyright Elsevier 2006.
as evidenced by rise in antistreptolysin O (ASO) titers during that period. Of note was the concomitant rise in heart-reactive antibody titers. The final infection was followed by a clinical recurrence of classical rheumatic cardi-tis complete with isolation of the organ-ism, elevated heart-reactive antibodies, and acute-phase reactants eleven years after the initial attack.
2.
Sera from
patients with ARF also con-tain increased levels of antibodies to both myosin
and tropomyosin, as com-pared with sera from patients with pha-ryngeal
streptococcal infections that do not develop ARF. These myosin-affinity
purified antibodies also cross-react with M protein moieties (known to share
amino acid homology with myosin), suggesting this molecule could be the
antigenic stimulus for the production of myosin antibodies in these sera.
3.
Finally,
as indicated earlier, autoim-mune antibodies are a prominent find-ing in
another major clinical manifes-tation of ARF, namely, Sydenham’s chorea, and
these antibodies are directed against the cells of the caudate nucleus. The
titers of this antibody cor-respond with clinical disease activity.
In all cases in which
autoreactive anti-bodies are seen (heart, brain, cardiac valves, kidney), they
can be absorbed with streptococcal antigens, notably
At a
cellular level, there is now ample evidence for the presence of both
lym-phocytes and macrophages at the site of pathological damage in the heart
valves in patients with ARF (see Figure 12.6). The cells are predominantly CD4+
helper lymphocytes during acute stages of the disease (4:1). The ratio of CD4+/CD8+
lym-phocytes (2:1) more closely approximates the normal ratio in chronic
valvular speci-mens. A majority of these cells express DR antigens. A
potentially important finding has been the observation that macrophage-like
fibroblasts present in the diseased valves express DR antigens and might be the
antigen-presenting cells for the CD4+ lymphocytes. Increased
cellular reactiv-ity to streptococcal antigens has also been noted in the
peripheral blood mononuclear cell preparations of ARF patients when compared
with these cells isolated from
Composition of the Mononuclear Celllular
Infiltrates in Acute and Chronic Active Rheumatic Valvulitis
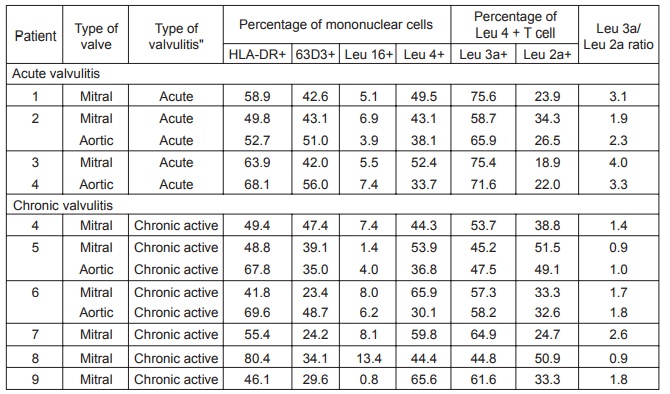
Figure 12.6 Composition of the mononuclear cellular infiltrates in acute and chronic rheumatic valvulitis. Note the increased CD4/CD8 ratios in acute cases of ARF compared with the more normal CD4/CD8 ratios in chronic more inactive disease patients.
nephritis patients. This abnormal reactiv-ity peaks
at six months after the attack but may persist for as long as two years after
the initial episode. Once again, the reactiv-ity was specific only for those
strains asso-ciated with ARF, suggesting an abnormal humoral and cellular
response to strepto-coccal antigens unique to rheumatic fever– associated
streptococci.
The observation that lymphocytes obtained from
experimental animals sensi-tized to cell membranes but not cell walls are
specifically cytotoxic for syngeneic embryonic cardiac myofibers in vitro
fur-ther strengthens support for the potential pathologic importance of these T
cells. In humans, normal mononuclear cells primed in vitro by M protein
molecules from a rheumatic fever–associated strain are also cytotoxic for
myofibers, but specificity solely for cardiac cells was lacking in the human
studies. Similar studies have not yet been performed using lymphocytes from
active ARF patients.
It has been known for years that often the early
symp-toms of chorea may present as emotional or behavioral changes in the
patient and only later do the choreiform motor symp-toms appear. Years after
the choreiform symptoms subsided a number of chorea patients presented with
behavioral disor-ders such as tics or obsessive-compulsive disorders (OCD).
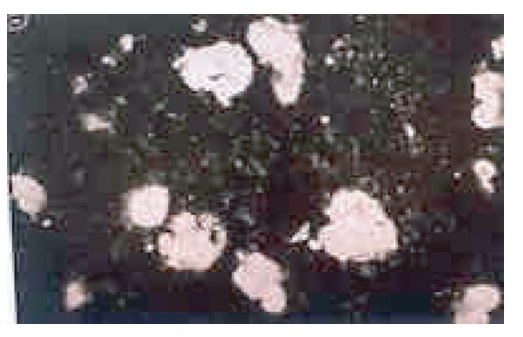
Figure 12.7 Immunofluorescent
micrograph of frozen sections of normal human caudate nucleus showing positive staining of large
neurons. The serum used was obtained from a child with prolonged active chorea.
These earlier observations coupled with the known
presence of antibrain antibodies in the sera of Sydenham’s chorea patients (see
Figure 12.7) raised the question of whether a prior streptococcal infection (or
infection with other microbes) might induce antibodies cross-reactive with
brain antigen(s) involved in neural path-ways associated with behavior. Two
recent papers indicate a strong association of the D8/17 B-cell marker with
children with OCD. While Swedo and colleagues selected patients on the basis of
a strong history of prior streptococcal infections, Murphy and colleagues noted
a strong association of the marker with OCD patients without a his-tory of
streptococcal infections. These pre-liminary studies suggest that streptococci
and probably other microbes may induce antibodies, which functionally disrupt
the basal ganglia pathways leading not only to classical chorea but also to
other behav-ioral disorders in these children without evidence of classical
chorea.
Second, the use of the D8/17 monoclo-nal antibody has also proved helpful in the differential diagnosis of ARF from other disorders.
In our hands, all rheumatic fever patients express abnormal levels of
D8/17-positive B cells, especially during the acute attack. In those cases
where the diagnosis of ARF has been doubtful, pres-ence of elevated levels of
D8/17-positive B cells has proven to be helpful in estab-lishing the correct
diagnosis.
Related Topics