Chapter: Essential Clinical Immunology: Immunological Aspects of Cardiac Disease
Molecular Mimicry in Chagas’ Disease
MOLECULAR MIMICRY IN CHAGAS’ DISEASE
Molecular mimicry of host antigens by parasite
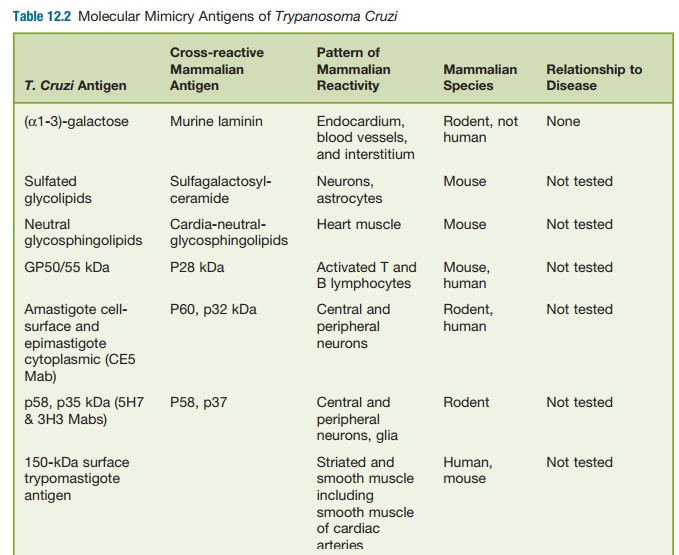
antigens has been found to gener-ate autoantibodies that are found in chronic T. cruzi infection (See table 12.2).
Autoim-mune pathogenesis by molecular mimicry requires breakdown in tolerance
of the immune system to self-antigens. A break-down in tolerance could occur in
response to chronic T. cruzi
infection or because of the polyclonal lymphocyte proliferative response that
occurs early after infection. Although many molecular mimicry epit-opes have
been described, only a few have been shown to correlate with disease in chronic
T. cruzi infection.
For example, high levels of antibod-ies to rodent
endocardium, blood vessels, and interstitium (EVI) have been found in the sera
of T. cruzi–infected individu-als and
the mammalian host and were absorbed by epimastigote antigens, thus
demonstrating cross-reactivity. However, these EVI antigens shown to be
directed against α-galactose epitopes were
spe-cific for murine laminin and not human laminin. Furthermore, anti-EVI
antibodies (α-galactose/laminin) or other
autoanti-bodies have not been shown to transfer disease to noninfected animals.

Perhaps more interesting (in terms of the organs
infected) has been a protein found on the surface of trypomastigotes in
asso-ciation with the flagellum, Fl-160. This pro-tein molecularly mimics
myenteric plexus and peripheral neurons. These antibodies are found in 30
percent of individuals with chronic Chagas’ disease; yet, they do not appear to
correlate with disease activity in these patients. In addition, T-cell
responses to this protein have not been seen in indi-viduals with Chagas’
disease, and passive transfer of these immune T-cells was not consistently
effective.
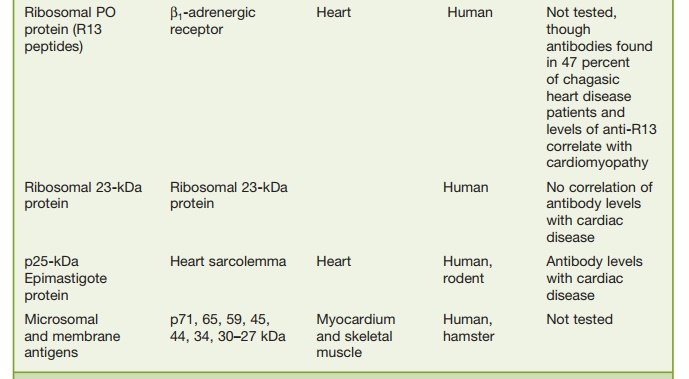
Another peptide of interest has been the ribosomal
Po protein (R13 peptide), which has been shown to cross-react with a functional
protein on human B1-adenergic receptors. Antibodies to this receptor via
immunization with the R13 peptide have led to electrocardiographic (ECG)
changes in mice that are similar to those seen in chronic Chagas’ carditis but
no changes in digestive symptoms have been noted.
Antibodies to an interesting T. cruzi protein called B13 is seen in the sera of all patients
with chronic Chagas’ cardio-myopathy and this antibody cross-reacts with human
cardiac myosin but in only 17 percent of asymptomatic individu-als.
Furthermore, T cells directed to both B13 and myosin have been detected in
biopsy specimens from persons with chronic Chagas’ cardiomyopathy. These
results suggest that antibody and T cells directed to cardiac myosin may be
patho-genic in chronic Chagas’ cardiomyopathy. However, in animal models
transfer of anti-body and T cells directed to myosin have not been reported to
lead to pathogenic changes. Perhaps the mouse model may not be correct and as
in rheumatic fever the introduction of myosin in the Lewis rat may be a better
animal model.
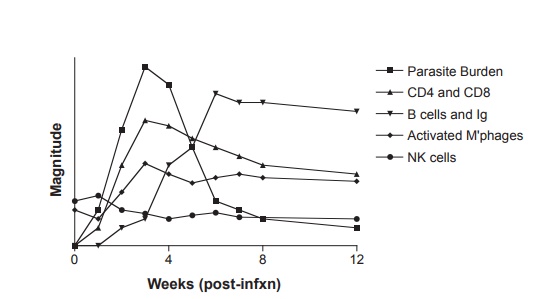
Figure 12.8 Relative magnitude of immune response mediators and parasite burden during acute Trypanosoma cruzi infection in the mouse. Adapted with permission from Buckner FS, Van Voorhis WC. Immune response to Trypanosoma cruzi: control of infection and pathogenesis of Chagas’ disease. In: Cunningham MW, Fujinami RS, eds. Effects of Microbes on the Immune System. Philadelphia, PA: Lippincott-Raven Press; 2000:5 69–591.
Related Topics