Chapter: Essential Microbiology: The Control of Microorganisms
Sterilisation - Control of Microorganisms
Sterilisation
One of the oldest forms of antimicrobial treatment is that of heating, and in most cases this remains the pre-ferred means of sterilisation, provided that it does not cause damage to the material in question. The benefits of boiling drinking water have been known at least since the 4th century BC, when Aristotle is said to have ad-vised Alexander the Great to order his troops to take this precaution. This of course was many centuries before the existence of microorganisms had been demonstrated or perhaps even suspected.
Sterilisation by heat
Boiling at 100 ◦ C for 10 minutes is usually enough to achieve sterility, provided that organisms are not present in high concentrations; in fact most bacteria are killed at about 70 ◦ C. If, however, endospores of certain bacteria (notably Bacillus and Clostridium)
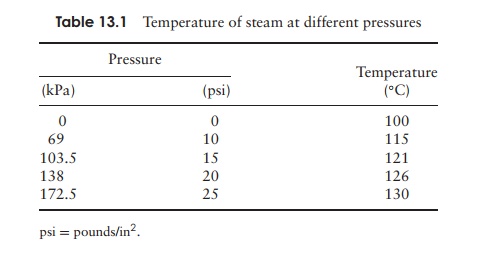
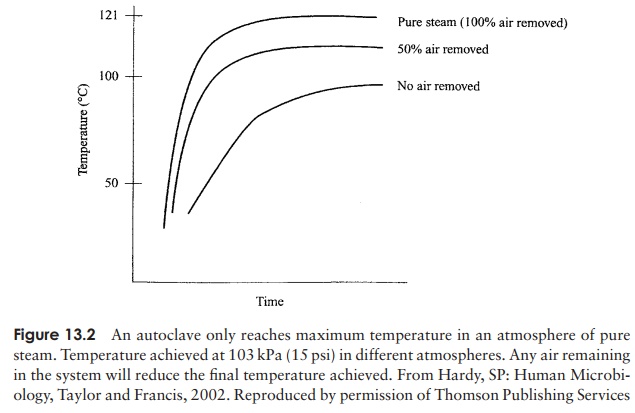
are present, they can resist boiling, sometimes for several hours. As we saw, the causative agents of some particularly nasty conditions, such as botulism and tetanus, are members of this group. In order to destroy the heat resistant endospores, heating beyond 100 ◦ C is required, and this can be achieved by heating under pressure in a closed vessel (Table 13.1). A typical laboratory treatment is 15 minutes at a pressure of 103 kpa (15 psi), raising the temperature of steam to 121 ◦ C. This is carried out in anautoclave, which is, to all intents and purposes, a large-scale pressure cooker (Figure 13.1). Air is driven out of the system so that the atmosphere is made up entirely of steam; the desired temperature will not be reached if this is not achieved (Figure 13.2). Large loads, or large volumes of liquids, may need a longer treatment time in order for
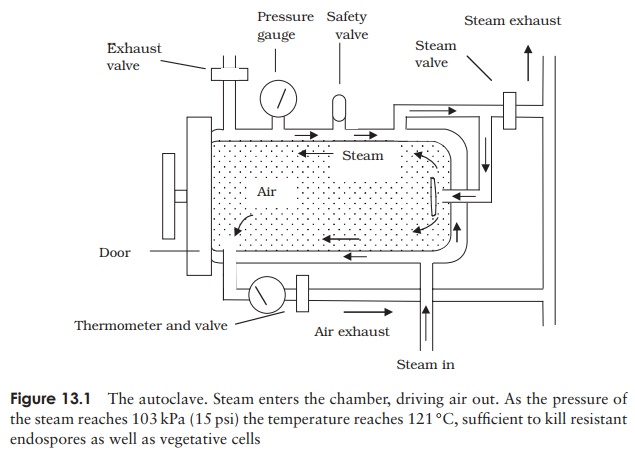
the heat to penetrate throughout. Modern autoclaves include probes designed to assess the temperature within a load rather than that of the atmosphere. Sometimes, spores of Bacillus stearothermophilus are introduced into a system along with the material to be sterilised; if subsequent testing shows that the spores have all been destroyed, it is reasonable to assume that the system has also destroyed any other biological entity present. Special tape which changes colour if the necessary temperature is reached can act as a more convenient but less reliable indicator.
An effect similar to that achieved by autoclaving can be obtained by a method called intermittent steaming or tyndallisation (after the Irish physicist John Tyndall, who was one of the first to demonstrate the existence of heat-resistant microbial forms). This is used for those substances or materials that might be damaged by the high tempera-tures used in autoclaving. The material is heated to between 90 and 100 ◦ C for about 30 minutes on each of three successive days, and left at 37 ◦ C in the intervening periods. Vegetative cells are killed off during the heating period, and during the 37 ◦ C incubation, any endospores that have survived will germinate. Once these have grown into more vegetative cells, they too are killed in the next round of steam treatment. Clearly this is quite a long-winded procedure, and it is therefore reserved for those materials which might be harmed by steam sterilisation.
High temperatures can cause damage to the taste, texture and nutritional value of many food substances and in such instances, it is sufficient to destroy vegetative cells by a process of pasteurisation (among his many other achievements, Pasteur demonstrated that the microbial spoilage of wines could be prevented by short periods of heating). Milk was traditionally pasteurised by heating large volumes at 63 ◦ C for 30 minutes, but the method employed nowadays is to pass it over a heat exchanger at 72 ◦ C for 15 seconds (HTST – high temperature, short time). This is not sterilisation as such, but it ensures the destruction of disease-causing organisms such as Brucella abortus andMycobacterium tuberculosis, which at one time were frequently found in milk, as wellas significantly reducing the organisms that cause food spoilage, thus prolonging the time the milk can be kept. Some protocols exceed these minimum values in order to reduce further the microbial content of the milk. One type of milk on sale in the shops is subjected to more extreme heating regimes; this is ‘UHT’ milk, which can be kept for several weeks without refrigeration, though many find that this is achieved at some cost to its palatability! It is heated to ultra high temperatures (150 ◦ C) for a couple of seconds using superheated steam. The product is often referred to as being ‘sterilised’, but this is not true in the strictest sense. Milk is not the only foodstuff to be pasteurised; others such as beer, fruit juices and ice cream each has its own time/temperature combination.
All the above methods employ a combination of heat and moisture to achieve their effect; the denaturation of proteins, upon which these methods depend, is enhanced in the presence of water. Heat is more readily transferred through water than through air, and the main reason that endospores are so resistant is because of their very low water content. In some situations however, it is possible to employ dry heat, using an oven to sterilise metal instruments or glassware for example. It is a more convenient procedure, but a higher temperature (160–170 ◦ C) and longer exposure time (2 hours) are required. Dry heat works by oxidising (‘burning’) the cell’s components. Microorganisms are quite literally burnt to destruction by the most extreme form of dry heat treatment, incineration. Soiled medical dressings and swabs, for example are potentially hazardous, and are destroyed in this way at many hundreds of degrees Celsius. As we saw, sterilising the loops and needles used to manipulate microorganisms by means of flaming is a routine part of aseptic procedures.
Sterilisation by irradiation
Certain types of irradiation are used to control the growth of microorganisms. These include both ionising and non-ionising radiation.
The most widely used form of non-ionising radiation is ultraviolet (UV) light. Wave-lengths around 260 nm are used because these are absorbed by the purine and pyrimidine components of nucleic acids, as well as certain aromatic amino acids in proteins. The absorbed energy causes a rupture of the chemical bonds, so that normal cellular func-tion is impaired. You will recall that UV light causes the formation of thymine dimers (Figure 11.21), where adjacent thymine nucleotides on the same strandare linked together, inhibiting DNA replication. Although many bacteria are capable of repairing this damage by enzyme-mediated photoreac-tivation, viruses are much more susceptible. UV lamps are commonly found in food preparation areas, operat-ing theatres and specialist areas such as tissue culture facilities, where it is important to prevent contamina-tion. Because they are also harmful to humans (particu-larly the skin and eyes), UV lamps can only be operated in such areas when people are not present. UV radia-tion has very poor penetrating powers; a thin layer of glass, paper or fabric is able to impede the passage ofthe rays. The chief application is therefore in the sterilisation of work surfaces and the surrounding air, although it is increasingly finding an application in the treatment of water supplies.
Ionising radiations have a shorter wavelength and much higher energy, giving them greater penetrating powers. The effect of ionising radiations is due to the production of highly reactive free radicals, which disrupt the structure of macromolecules such as DNA and proteins. Surgical supplies such as syringes, catheters and rubber gloves are commonly sterilised employing gamma (γ ) rays from the isotope cobalt 60 (60Co).
Gamma radiation has been approved for use in over 40 countries for the preservation of food, which it does not only by killing pathogens and spoilage organisms but also by inhibiting processes that lead to sprouting and ripening. The practice has aroused a lot of controversy, largely due to concerns about health and safety, although the first patent applications for its use date back nearly a hundred years! Although the irradiated product does not become radioactive, there is a general suspicion on the part of the public about anything to do with radiation, which has led to its use on food being only very gradually accepted by consumers. In this respect Europe lags behind the USA, where during the 1990s a positive attitude towards irradiation of food both by professional bodies and the media has led to a more widespread acceptance of the technology. Gamma radiation is used in situations where heat sterilisation would be inappropriate, because of undesirable effects on the texture, taste or appearance of the product. This mainly relates to fresh produce such as meat, poultry, fruit and vegetables. Irradiation is not suitable for some foodstuffs, such as those with a high fat content, where unpleasant tastes and odours result. Ionising radiations have the great advantage over other methods of sterilisation that they can penetrate packaging.
Filtration
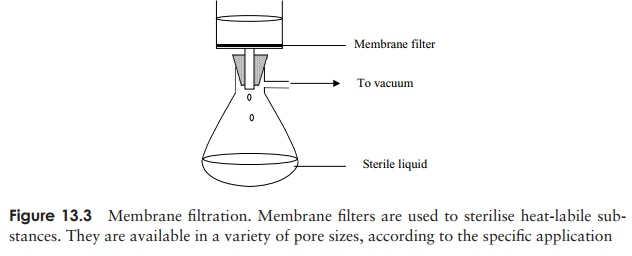
Many liquids such as solutions of antibiotics or certain components of culture media become chemically altered at high temperatures, so the use of any of the heat regimes described above is not appropriate. Rather than killing the microorganisms, an alter-native approach is simply to isolate them. This can be done for liquids and gases by passing them through filters of an appropriate pore size. Filters used to be made from materials such as asbestos and sintered glass, but have been largely replaced by mem-brane filters, commonly made of nitrocellulose or polycarbonate (Figure 13.3). These can be purchased ready-sterilised and the liquid passed through by means of pressure or suction. Supplies of air or other gases can also be filter-sterilised in this way. A pore size of 0.22 µm is commonly used; this will remove bacteria plus, of course, anything bigger, such as yeasts; however, mycoplasma and viruses are able to pass through pores of this size. With a pore size 10 times smaller than this, only the smallest of viruses can pass through, so it is important that an appropriate pore size is chosen for any given task. A drawback with all filters, but especially those of a small pore size, is that they can become clogged easily. Filters in general are relatively expensive, and are not the preferred choice if alternative methods are available.
High efficiency particulate air (HEPA) filters create clean atmospheres in areas such as operating theatres and laboratory laminar-flow hoods.
Sterilisation using ethylene oxide
Generally, chemical methods achieve only disinfection ; the use of the gas ethylene oxide, however, is effective against bacteria, their spores and viruses. It is used for sterilising large items of medical equipment, and materials such as plastics that would be damaged by heat treatment. Ethylene oxide is particularly effective in sterilising items such as dressings and mattresses, due to its great powers of penetration. In the food industry, it is used as an antifungal fumigant, for the treatment of dried fruit, nuts and spices. The materials to be treated are placed in a special chamber which is sealed and filled with the gas in a humid atmosphere at 40–50 ◦ C for several hours. Ethylene oxide is highly explosive, so it must be used with great caution; its use is rendered safer by administering it in admixture (10 per cent) with a non-flammable gas such as carbon dioxide. It is also highly toxic, so all items must be thoroughly flushed with sterile air following treatment to remove any trace of it. Ethylene oxide is an alkylating agent; it denatures proteins by replacing labile hydrogens such as those on sulphydryl groups with a hydroxyl ethyl radical (Figure 13.4).
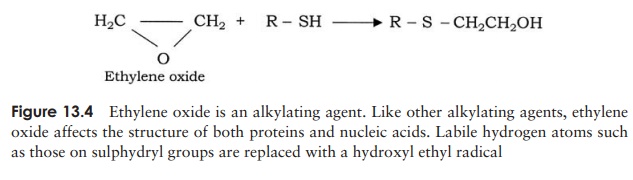
Related Topics