Chapter: Genetics and Molecular Biology: Regulation of Mating Type in Yeast
Sterile Mutants, Membrane Receptors and G Factors
Sterile Mutants, Membrane Receptors and G
Factors
One method for exploring the complexity of the
mating-type system is to determine the range of defective mutant types that can
be isolated. Additionally, mutants greatly facilitate biochemical studies, as
they often permit associating a particular biochemical defect with a response
of the system. Sterile mutants are particularly easy to isolate. Wild-type haploid
cells cease growth in the presence of the opposite mating type sex pheromone.
Therefore, any cells that continue growth must be defective in their detection
of or response to the sex pheromones. Suitable pheromone for this selection can
be obtained from the medium by growing α mating type cells to a high density, although chemically synthesized α type pheromone is now cheaper and more pure. The a
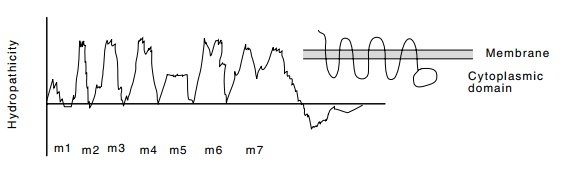
Figure
16.13 A representation of the
hydropathicity plot of mating factorreceptor. These plots are running averages
of the hydropathicity of seven amino acids. A large hydropathicity indicates a
membrane spanning region of the protein.
pheromone cannot easily be synthesized because it
is only active when when post-translationally modified to a form not easily
synthesized invitro.
Amongst the sterile mutants which can be found by
resistance to growth factor inhibition are those lacking the receptor for
mating-type pheromone. These mutants can be identified by their lack of binding
of radioactive factor to intact cells. Of course, once such defective mutants
are found, the gene responsible can be isolated. This has been done, and the
sequences of the a and α receptors turn out to be similar to a class of
membrane receptors including both rhodopsin and β-adrenergic receptor. These proteins possess seven hydrophobic regions
that cross the membrane seven times (Fig. 16.13). In general these proteins
couple extracellular events to intracellular actions.
In higher cells the next protein in the chain from
membrane receptors to alterations in gene expression is called G protein
because it normally binds GDP. In the absence of stimulation, one of the three
subunits of G binds GDP. Upon stimulation, the α subunit of G binds GTP and
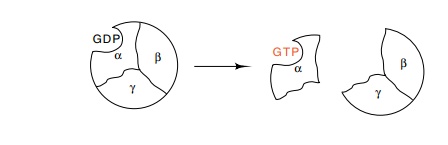
dissociates from the β and γ subunits. It has been difficult in higher cells to determine whether it is the α subunit that activates or the β-γ dimer that activates the next step in a signaling pathway. In yeast, however, it is quite clear that the β-γ pair play an important role in regulation, for an absence of the α subunit or an excess of β and γ generates the same response as exposure to mating factor. Judged on the similarity of their amino acid sequences to proteins with known activities, the later pro-teins in the signal chain are likely to be protein kinases. The final protein in the signaling chain is a transcription factor which binds and activates transcription of the appropriate genes.
Related Topics