Chapter: Clinical Dermatology: The function and structure of the skin
Skin Epidermis
Epidermis
The
epidermis is formed from many layers of closely packed cells, the most
superficial of which are flattened and filled with keratins; it is therefore a
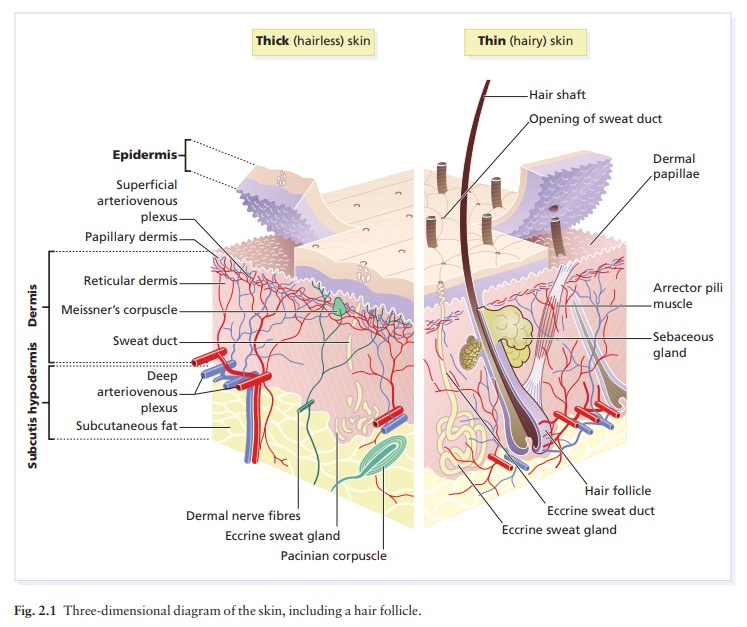
stratified squam-ous epithelium. It adheres to the dermis partly by the
interlocking of its downward projections (epidermalridges or pegs)
with upward projections of the dermis(dermal papillae)
(Fig. 2.1).
The epidermis contains no blood
vessels. It varies in thickness from less than 0.1 mm on the eyelids to nearly
1 mm on the palms and soles. As dead surface squames are shed (accounting for
some of the dust in our houses), the thickness is kept constant by cells
dividing in the deepest (basal or germinative) layer. A generated cell moves, or
is pushed by underlying mitotic activity, to the surface, passing through the prickle and granular
cell layers before
dying in the horny
layer.
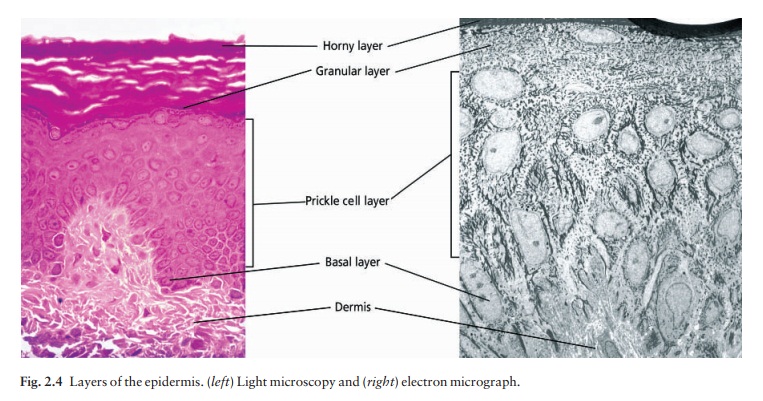
Thejourney from the basal layer to the surface (epidermal turnover or transit
time) takes about 60 days. During this time the appearance of the cell changes.
A vertical section through the epidermis summarizes the life history of a
single epidermal cell (Fig. 2.2).
The basal
layer,
the deepest layer, rests on a base-ment membrane, which attaches it to the dermis. It
is a single layer of columnar cells, whose basal surfaces sprout many fine
processes and hemidesmosomes, anchoring them to the lamina
densa
of the basement membrane.
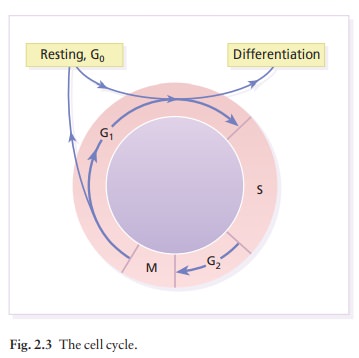
In normal skin some 30% of basal cells are prepar-ing for division (growth fraction). Following mitosis, a cell enters the G1 phase, synthesizes RNA and protein, and grows in size (Fig. 2.3). Later, when the cell is triggered to divide, DNA is synthesized (S phase) and chromosomal DNA is replicated. A short postsynthetic (G2) phase of further growth occurs before mitosis (M). DNA synthesis continues through the S and G2 phases, but not during mitosis. The G1 phase is then repeated, and one of the daughter cells moves into the supra-basal layer. It then differentiates (Fig. 2.2), having lost the capacity to divide, and synthesizes keratins. Some basal cells remain inactive in a so-called G0 phase but may re-enter the cycle and resume proliferation.
The cell
cycle time in normal human skin is controversial; estimates of 50–200 h reflect
differing views on the duration of the G1
phase. Stem cells reside amongst these basal cells and amongst the cells
of the external root sheath of the hair follicle at the level of attachment of
the arrector pili muscle but cannot be identified by histology. These cells
divide infrequently, but can generate new proliferative cells in the epidermis
and hair follicle in response to damage.
Keratinocytes
The spinous or prickle cell layer (Fig. 2.4) is composed of keratinocytes. These differentiating cells, which synthesize keratins, are larger than basal cells. Keratinocytes are firmly attached to each other by small interlocking cytoplasmic processes, by abundant desmosomes and by an intercellular cement of glycoproteins and lipo-proteins. Under the light microscope, the desmosomes look like ‘prickles’. They are specialized attachment plaques that have been characterized biochemically. They contain desmoplakins, desmogleins and desmocollins. Autoantibodies to these proteins are found in pemphigus, when they are responsible for the detachment of keratinocytes from one another and so for intraepidermal blister formation. Cytoplasmic continuity between keratinocytes occurs at gap junctions, specialized areas on opposing cell walls. Tonofilaments are small fibres running from the cytoplasm to the desmosomes.
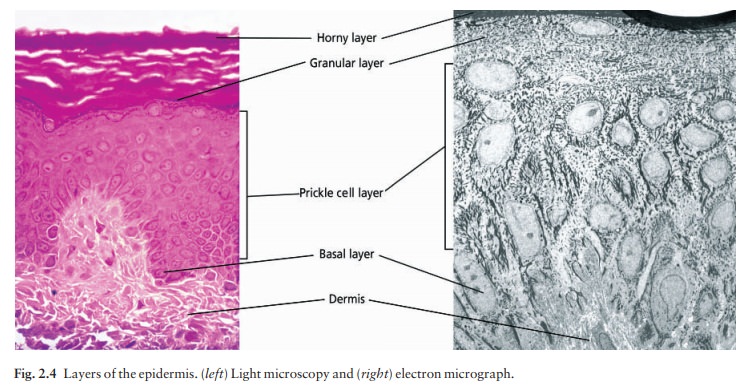
They are more numer-ous in cells of the spinous layer than
of the basal layer, and are packed into bundles called tonofibrils.
Many lamellar
granules (otherwise known as membrane-coating granules, Odland bodies
or keratinosomes), derived from the Golgi apparatus, appear in the super-ficial
keratinocytes of this layer. They contain poly-saccharides, hydrolytic enzymes
and, more importantly, stacks of lipid lamellae composed of phospholipids,
cholesterol and glucosylceramides. Their contents are discharged into the
intercellular space of the granular cell layer to become precursors of the
lipids in the intercellular space of the horny layer (see Barrierfunction
below).
Cellular
differentiation continues in the granular layer, which normally consists of two
or three layers of cells that are flatter than those in the spinous layer, and
have more tonofibrils. As the name of the layer implies, these cells contain
large irregular basophilic granules of keratohyalin,
which merge with tonofibrils. These keratohyalin granules contain proteins,
includ-ing involucrin, loricrin and profilaggrin, which is cleaved into
filaggrin by specific phosphatases as the granular cells move into the horny
layer.
As
keratinocytes migrate out through the outer-most layers, their keratohyalin
granules break up and their contents are dispersed throughout the cytoplasm,
leading to keratinization and the formation of a thick and tough peripheral
protein coating called the hornyenvelope. Its structural proteins include
loricrin andinvolucrin, the latter binding to ceramides in the sur-rounding
intercellular space under the influence of transglutaminase. Filaggrin,
involucrin and loricrin can all be detected histochemically and are useful as
markers of epidermal differentiation.
The
horny
layer (stratum corneum) is made of piled-up layers of flattened
dead cells (corneocytes)a the bricksastuck together by lipidsathe mortarain the
intercellular space. The corneocyte cytoplasm is packed with keratin filaments,
embedded in a matrix and enclosed by an envelope derived from the keratohyalin
granules. This envelope, along with the aggregated keratins that it encloses,
gives the corneocyte its tough-ness, allowing the skin to withstand all sorts
of chem-ical and mechanical insults. Horny cells normally have no nuclei or
intracytoplasmic organelles, these having been destroyed by hydrolytic and
degrading enzymes found in lamellar granules and the lysosomes of granular
cells.
Keratinization
All
cells have an internal skeleton made up of microfila-ments (7 nm diameter;
actin), microtubules (20–35 nm diameter; tubulin) and intermediate filaments
(10 nm diameter). Keratins (from the Greek keras meaning
‘horn’) are the main intermediate filaments in epithe-lial cells and are
comparable to vimentin in mesenchy-mal cells, neurofilaments in neurones and
desmin in muscle cells. Keratins are not just a biochemical curiosity, as
mutations in their genes cause a number of skin diseases including simple
epidermolysis bul-losa and bullous
ichthyosiform erythroderma.
The
keratins are a family of more than 30 proteins, each produced by different
genes. These separate into two gene families: one responsible for basic and the
other for acidic keratins. The keratin polypeptide has a central helical
portion with a non-helical N-terminal head and C-terminal tail. Individual
keratins exist in pairs so that their double filament always consists of one
acidic and one basic keratin polypeptide. The inter-twining of adjacent
filaments forms larger fibrils.
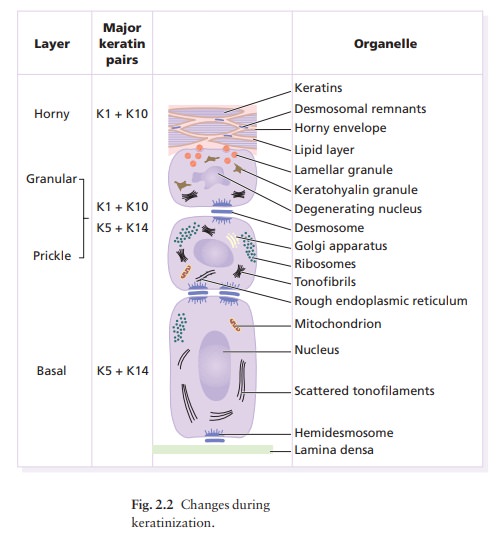
Different
keratins are found at different levels of the epidermis depending on the stage
of differenti-ation and disease; normal basal cells make keratins 5 and 14, but
terminally differentiated suprabasal cells make keratins 1 and 10 (Fig. 2.2).
Keratins 6 and 16 become prominent in hyperproliferative states such as
psoriasis.
During
differentiation, the keratin fibrils in the cells of the horny layer align and
aggregate, under the influence of filaggrin. Cysetine, found in keratins of the
horny layer, allows cross-linking of fibrils to give the epidermis strength to
withstand injury.
Cell cohesion and desquamation
Firm
cohesion in the spinous layer is ensured by ‘stick and grip’ mechanisms. A
glycoprotein intercellular sub-stance acts as a cement, sticking the cells
together, and the intertwining of the small cytoplasmic processes of the
prickle cells, together with their desmosomal attachments, accounts for the
grip. The cytoskeleton of tonofibrils also maintains the cell shape rigidly.
The
typical ‘basket weave’ appearance of the horny layer in routine histological
sections is artefactual and deceptive. In fact, cells deep in the horny layer
stick tightly together and only those at the surface flake off; this is in part
caused by the activity of cholesterol sulphatase. This enzyme is deficient in
X-linked recessive ichthyosis, in which poor shedding leads to the piling up of
corneocytes in the horny layer. Desquamation is normally responsible for the
removal of harmful exogenous substances from the skin surface. The cells lost
are replaced by newly formed corneocytes; regeneration and turnover of the
horny layer is therefore continuous.
The epidermal barrier
The
horny layer prevents the loss of interstitial fluid from within, and acts as a
barrier to the penetration of potentially harmful substances from outside.
Solvent extraction of the epidermis leads to an increased per-meability to
water, and it has been known for years that essential fatty acid deficiency causes
poor cutan-eous barrier function. These facts implicate ceramides, cholesterol,
free fatty acids (from lamellar granules;), and smaller quantities of other
lipids, in cutaneous barrier formation. Barrier function is also impaired when
the horny layer is removed experiment-ally, by successive strippings with
adhesive tape, or clinically, by injury or skin disease. It is also decreased
by excessive hydration or dehydration of the horny layer and by detergents.
The rate of penetration of a substance through the epidermis is directly proportional to its concentration difference across the barrier layer, and indirectly pro-portional to the thickness of the horny layer. A rise in skin temperature aids penetration. A normal horny layer is slightly permeable to water, but relatively impermeable to ions such as sodium and potassium. Some other substances (e.g. glucose and urea) also penetrate poorly, whereas some aliphatic alcohols pass through easily. The penetration of a solute dis-solved in an organic liquid depends mainly on the qualities of the solvent.
Epidermopoiesis and its regulation
Both
the thickness of the normal epidermis, and the number of cells in it, remain
constant, as cell loss at the surface is balanced by cell production in the
basal layer. Locally produced polypeptides (cytokines), growth factors and
hormones stimulate or inhibit epidermal proliferation, interacting in complex
ways to ensure homeostasis. Cytokines and growth factors (Table 2.2) are
produced by keratinocytes, Langerhans cells, fibroblasts and lymphocytes within
the skin. After these bind to high affinity cell surface receptors, DNA
synthesis is controlled by signal transduction, involving protein kinase C or inositol
phosphate. Catecholamines, which do not penetrate the surface of cells,
influence cell division via the adenosine 3′,
5′-cyclic
monophosphate (cAMP) second messenger system. Steroid hormones bind to receptor
proteins within the cytoplasm, and then pass to the nucleus where they
influence transcription.
Vitamin D synthesis
The
steroid 7-dehydrocholesterol, found in ker-atinocytes, is converted by sunlight
to cholecalciferol. The vitamin becomes active after 25-hydroxylation in the
kidney. Lack of sun and kidney disease can both cause vitamin D deficiency and
rickets.
Other cells in the epidermis
Keratinocytes
make up about 85% of cells in the epidermis, but three other types of cell are
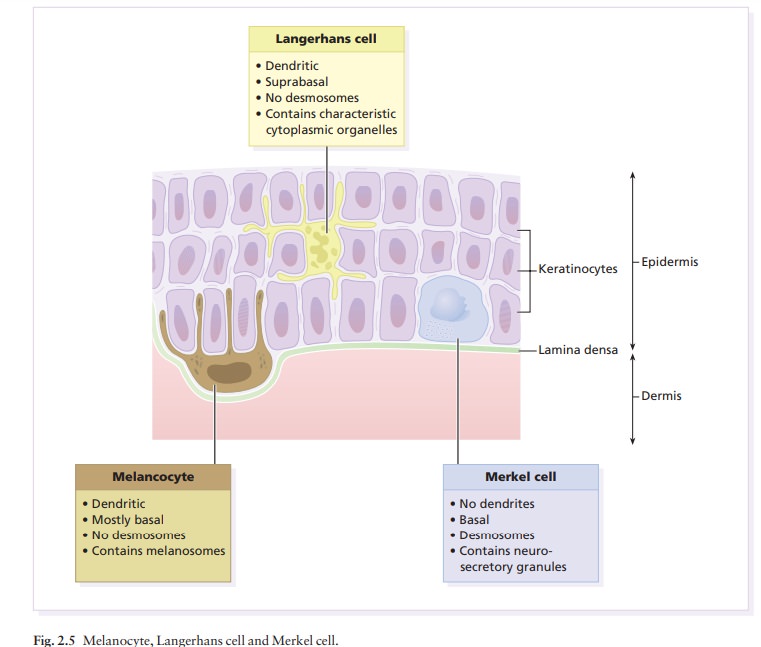
also found there: melanocytes, Langerhans cells and Merkel cells. (Fig.
2.5).Melanocytes are the only cells that can synthesize melanin. They migrate
from the neural crest into the basal layer of the ectoderm where, in human
embryos, they are seen as early as the eighth week of gestation. They are also
found in hair bulbs, the retina and pia arachnoid. Each dendritic melanocyte
associates with a number of keratinocytes, forming an ‘epidermal melanin unit’
(Fig. 2.5). The dendritic processes of melanocytes wind between the epidermal
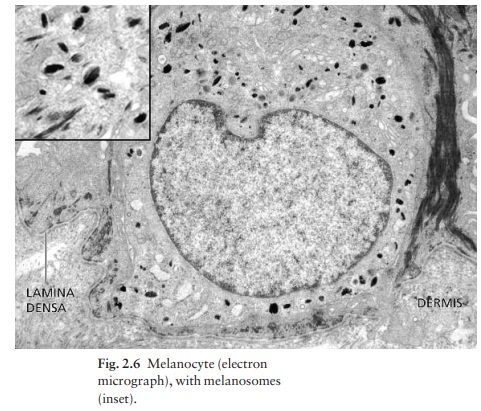
cells and end as discs in contact with them. Their cytoplasm contains discrete
organelles, the melanosomes,
containing vary-ing amounts of the pigment melanin (Fig. 2.6).
Langerhans cells
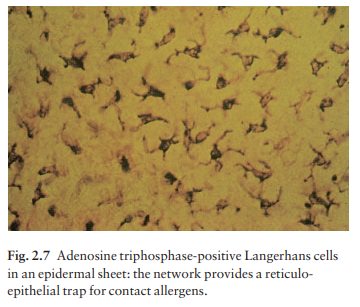
The Langerhans cell is a dendritic cell (Figs 2.5 and 2.7) like the melanocyte. It also lacks desmosomes and tonofibrils, but has a lobulated nucleus.
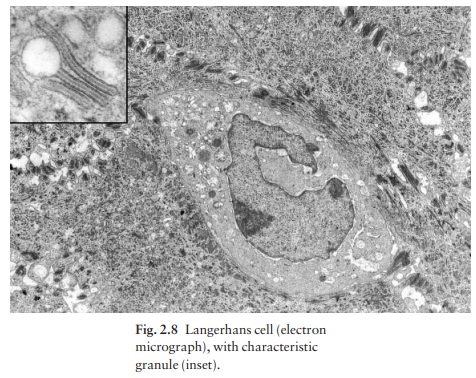
The specific granules
within the cell look like a tennis racket when seen in two dimensions in an
electron micrograph (Fig. 2.8), or like a sycamore seed when reconstructed in
three dimensions. They are plate-like, with a rounded bleb protruding from the
surface.
Langerhans
cells come from a mobile pool of pre-cursors originating in the bone marrow.
There are approximately 800 Langerhans cells per mm2
in human skin and their dendritic processes fan out to form a striking network
seen best in epidermal sheets (Fig. 2.7). Langerhans cells are alone among
epidermal cells in possessing surface receptors for C3b and the Fc por-tions of
IgG and IgE, and in bearing major histocompat-ibility complex (MHC) Class II
antigens (HLA-DR, -DP and -DQ). They are best thought of as highly
spe-cLangerhans cells have a key role in many immune reactions. They take up
exogenous antigen, process it and present it to T lymphocytes either in the
skin or in the local lymph nodes. They probably play a part in
immunosurveillance for viral and tumour antigens. In this way, ultraviolet
radiation can induce skin tumours both by causing mutations in the epidermal
cells, and by decreasing the number of epidermal Langerhans cells, so that
cells bearing altered antigens are not recognized or destroyed by the immune
system. Topical or systemic glucocorticoids also reduce the density of
epidermal Langerhans cells. The Langerhans cell is the principal cell in skin
allo-grafts to which the T lymphocytes of the host react during rejection;
allograft survival can be prolonged by depleting Langerhans cells.
Merkel cells
Merkel cells are found in normal epidermis (Fig. 2.5) and act as transducers for fine touch. They are non-dendritic cells, lying in or near the basal layer, and are of the same size as keratinocytes. They are con-centrated in localized thickenings of the epidermis near hair follicles (hair discs), and contain membrane-bound spherical granules, 80–100 nm in diameter, which have a core of varying density, separated from the membrane by a clear halo. Sparse desmosomes connect these cells to neighbouring keratinocytes. Fine unmyelinated nerve endings are often associated with Merkel cells, which express immunoreactivity for various neuropeptides .
Epidermal appendages
The
skin appendages are derived from epithelial germs during embryogenesis and,
except for the nails, lie in the dermis. They include hair, nails and sweat and
sebaceous glands.
Related Topics