Chapter: Clinical Dermatology: The function and structure of the skin
Hypersensitivity reactions in the skin
Hypersensitivity
reactions in the skin
Hypersensitivity
is the term given to an exaggerated or inappropriate immune reaction. It is
still helpful, if rather artificial, to separate these into four main types
using the original classification of Coombs and Gell. All of these types
underlie reactions in the skin.
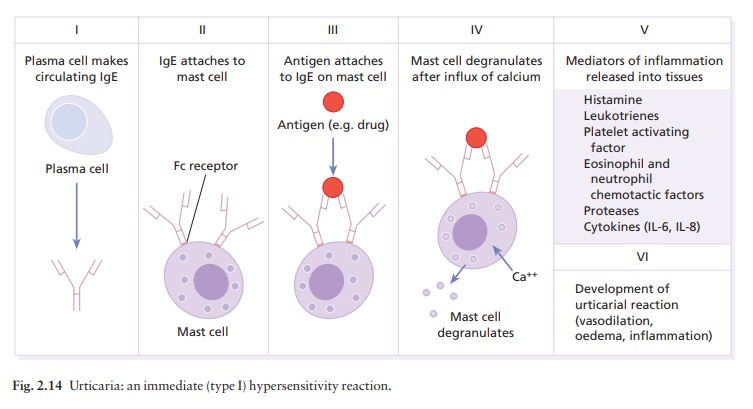
Type I: immediate hypersensitivity reactions
These
are characterized by vasodilatation and an out-pouring of fluid from blood
vessels. Such reactions can be mimicked by drugs or toxins, which act directly,
but immunological reactions are mediated by anti-bodies, and are manifestations
of allergy. IgE and IgG4 antibodies, produced by plasma cells in organs other
than the skin, attach themselves to mast cells in the dermis. These contain
inflammatory mediators, either in granules or in their cytoplasm. The IgE
anti-body is attached to the mast cell by its Fc end, so that the antigen
combining site dangles from the mast cell like a hand on an arm (Fig. 2.14).
When specific antigen combines with the hand parts of the immuno-globulin (the
antigen-binding site or Fab end), the mast cell liberates its mediators into
the surround-ing tissue. Of these mediators, histamine (from the granules) and
leukotrienes (from the cell membrane) induce vasodilatation, and endothelial
cells retract allowing transudation into the extravascular space. The
vasodilatation causes a pink colour, and the tran-sudation causes swelling.
Urticaria and angioedema are examples of
immediate hypersensitivity reactions occurring in the skin.
Antigen
may be delivered to the skin from the out-side (e.g. in a bee sting). This will
induce a swelling in everyone by a direct pharmacological action. However, some
people, with IgE antibodies against antigens in the venom, swell even more at
the site of the sting as the result of a specific immunological reaction. If
they are extremely sensitive, they may develop wheezing, wheals and
anaphylactic shock (see Fig. 22.5), because of a massive release of histamine
into the circulation.
Antigens
can also reach mast cells from inside the body. Those who are allergic to
shellfish, for example, may develop urticaria within seconds, minutes or hours
of eating one. Antigenic material, absorbed from the gut, passes to tissue mast
cells via the circulation, and elicits an urticarial reaction after binding to
specific IgE on mast cells in the skin.
Type II: humoral cytotoxic reactions
In
the main, these involve IgG and IgM antibodies, which, like IgE, are produced
by plasma cells and are present in the interstitial fluid of the skin. When
they meet an antigen, they fix and activate complement through a series of
enzymatic reactions that generate mediator and cytotoxic proteins. If bacteria
enter the skin, IgG and IgM antibodies bind to antigens on them. Complement is
activated through the classical pathway, and a number of mediators are
generated. Amongst these are the chemotactic factor, C5a, which attracts
polymorphs to the area of bacterial invasion, and the opsonin, C3b, which coats
the bacteria so that
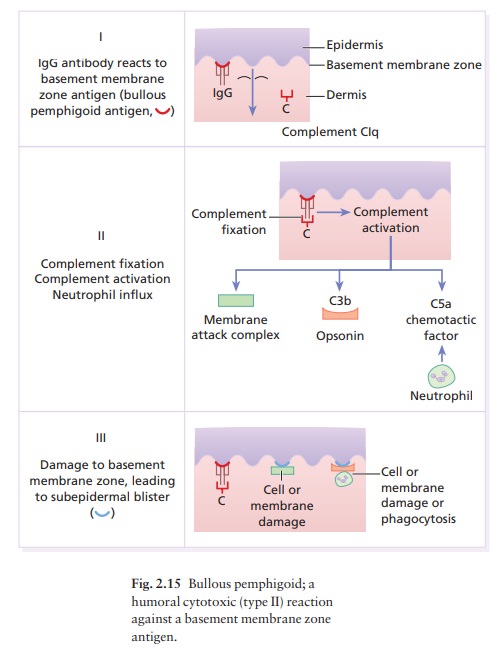
Under
certain circumstances, activation of complement can kill cells or organisms
directly by the ‘membrane attack complex’ (C5b6789) in the terminal complement
pathway. Complement can also be activated by bacteria directly through the
altern-ative pathway; antibody is not required. The bacterial cell wall causes
more C3b to be produced by the altern-ative pathway factors B, D and P
(properdin). Aggreg-ated IgA can also activate the alternative pathway.
Activation
of either pathway produces C3b, the pivotal component of the complement system.
Through the amplification loop, a single reaction can flood the area with C3b,
C5a and other amplification loop and terminal pathway components. Complement is
the mediator of humoral reactions.
Humoral
cytotoxic reactions are typical of defence against infectious agents such as
bacteria. However, they are also involved in certain autoimmune diseases such
as pemphigoid .
Occasionally,
antibodies bind to the surface of a cell and activate it without causing its
death or activating complement. Instead, the cell is stimul-ated to produce a
hormone-like substance that may mediate disease. Pemphigus is a blistering disease of skin in which this
type of reaction may be important.
Type III: immune complex-mediated reactions
Antigen
may combine with antibodies near vital tissues so that the ensuing inflammatory
response damages
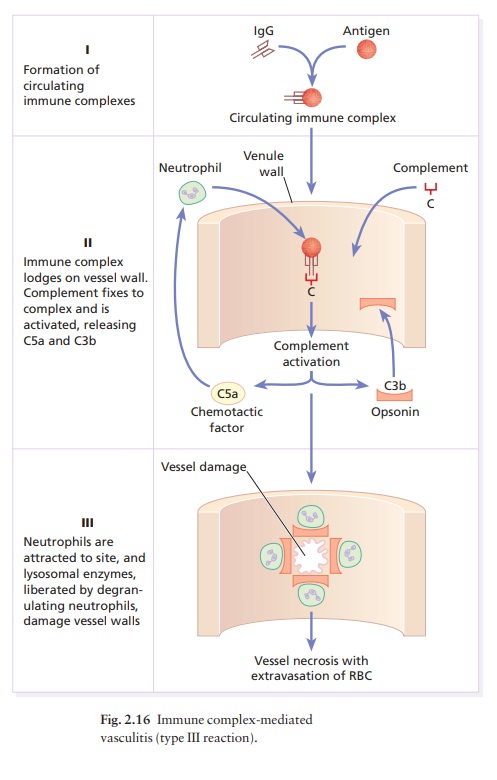
When an antigen is injected intradermally, it combines with appropriate
antibodies on the walls of blood vessels, complement is activated, and
polymor-phonuclear leucocytes are brought to the area (an Arthus reaction).
Degranulation of polymorphs liber-ates lysosomal enzymes that damage the vessel
walls.
Antigen–antibody complexes can also be formed in the circulation, move to the small vessels in the skin and lodge there (Fig. 2.16). Complement will then be activated and inflammatory cells will injure the vessels as in the Arthus reaction. This causes oedema and the extravasation of red blood cells (e.g. the palpable purpura that characterizes vasculitis; ).
Type IV: cell-mediated immune reactions
As
the name implies, these are mediated by lymphocytes rather than by antibodies.
Cell-mediated immune reac-tions are important in granulomas, delayed hypersensi-tivity
reactions, and allergic contact dermatitis. They probably also play a part in
some photosensitive dis-orders, in protecting against cancer, and in mediating
reactions to insect bites.
Allergic contact dermatitis
There
are two phases: during the induction phase naïve lymphocytes become sensitized
to a specific antigen; during the elicitation phase antigens entering the skin
are processed by antigen-presenting cells such as macrophages and Langerhans
cells (Fig. 2.17) and then interact with sensitized lymphocytes. The
lympho-cytes are stimulated to enlarge, divide and to secrete cytokines that
can injure tissues directly and kill cells or microbes.
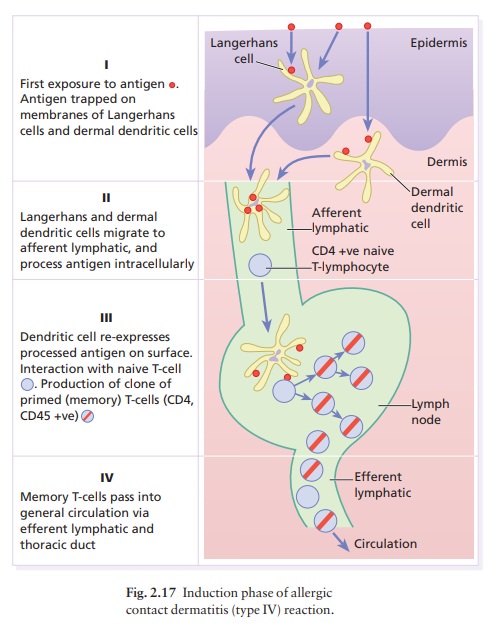
Induction
(sensitization) phase (Fig. 2.17)
When the epidermal barrier is breached, the immune system provides the second line of defence. Among the keratinocytes are Langerhans cells, highly specialized intraepidermal macrophages with tentacles that inter-twine among the keratinocytes, providing a net (Fig. 2.7) to ‘catch’ antigens falling down on them from the surface, such as chemicals or the antigens of microbes or tumours. During the initial induction phase, the antigen is trapped by a Langerhans cell which then migrates to the regional lymph node. To do this, it must retract its dendrites and ‘swim upstream’ from the prickle cell layer of the epidermis towards the base-ment membrane, against the ‘flow’ of keratinocytes generated by the epidermal basal cells.
Once in the dermis,
the Langerhans cell enters the lymphatic sys-tem, and by the time it reaches
the regional lymph node it will have processed the antigen, which is
re-expressed on its surface in conjunction with MHC Class II molecules. In the
node, the Langerhans cell mingles with crowds of lymphocytes, where it is most
likely to find a T cell with just the right T-cell receptor to bind its now
processed antigen. Helper (CD4+) T lymphocytes recognize antigen only in the presence of
cells bearing MHC Class II antigens, such as the Langerhans cell. The
interactions between surface molecules on a CD4+ T cell
and a Langerhans cell are shown in Fig. 2.12. When a T cell interacts with an
antigen-presenting cell carrying an antigen to which it can react, the T
lymphocyte divides. This division depends upon the persistence of antigen (and
the antigen-presenting cells that contain it) and the T-cell growth factor
interleukin-2 (IL-2). Eventually, a whole cadre of memory T cells is available
to return to the skin to attack the antigen that stimulated their
proliferation.
CD4+, CD45+ memory T
lymphocytes circulate between nodes and tissues via lymphatic vessels, the
thoracic duct, blood and interstitial fluid. They return to the skin aided by
‘homing molecules’ (cutaneous lymphocyte antigen, CLA) that guide their trip so
that they preferentially enter the dermis. In the absence of antigen, they
merely pass through it, and again enter the lymphatic vessels to return and
recirculate. These cells are sentinel cells (Fig. 2.18), alert for their own
special antigens. They accumulate in the skin if the host again encounters the
antigen that initially
stimulated
their production. This preferential circula-tion of lymphocytes into the skin
is a special part of the ‘skin immune system’ and reflects a selective
advantage for the body to circulate lymphocytes that react to skin and skin
surface-derived antigens.
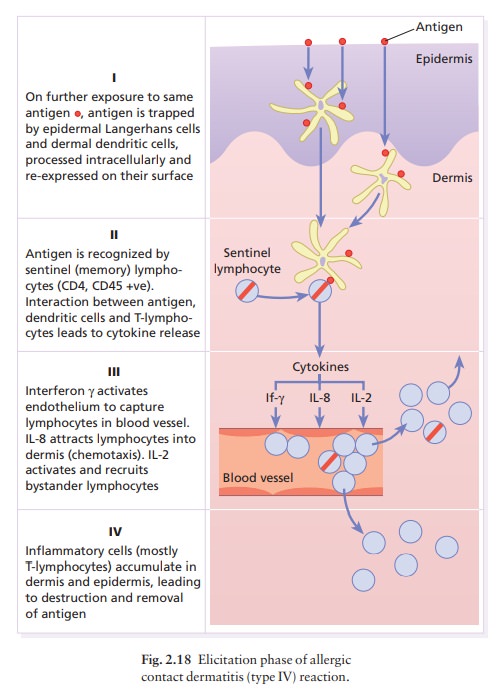
Elicitation
(challenge) phase (Fig. 2.18)
When
a T lymphocyte again encounters the antigen to which it is sensitized, it is
ready to react. If the antigen is extracellular, as on an invading bacterium,
toxin or chemical allergen, the CD4+ T-helper cells do the work. The
sequence of antigen processing by the Langerhans cell in the elicitation
reaction is similar to the sequence of antigen processing during the induction
phase, described above, that leads to the induction of immun-ity. The antigens
get trapped by epidermal Langerhans cells or dermal dendritic cells, which
process the anti-gen intracellularly before re-expressing the modified
antigenic determinant on their surfaces. In the elicita-tion reaction, the
Langerhans cells find appropriate T lymphocytes in the dermis, so most antigen
presenta-tion occurs there. The antigen is presented to CD4+ T cells
which are activated and produce cytokines that cause lymphocytes,
polymorphonuclear leucocytes and monocytes in blood vessels to slow as they
pass through dermal blood vessels, to stop and emigrate into the dermis causing
inflammation (Fig. 2.18). Helper or cytotoxic lymphocytes help to stem the
infection or eliminate antigen and polymorphonuclear leucocytes engulf antigens
and destroy them. The traffic of inflam-matory cells in the epidermis and
dermis is determined not only by cytokines produced by lymphocytes, but also by
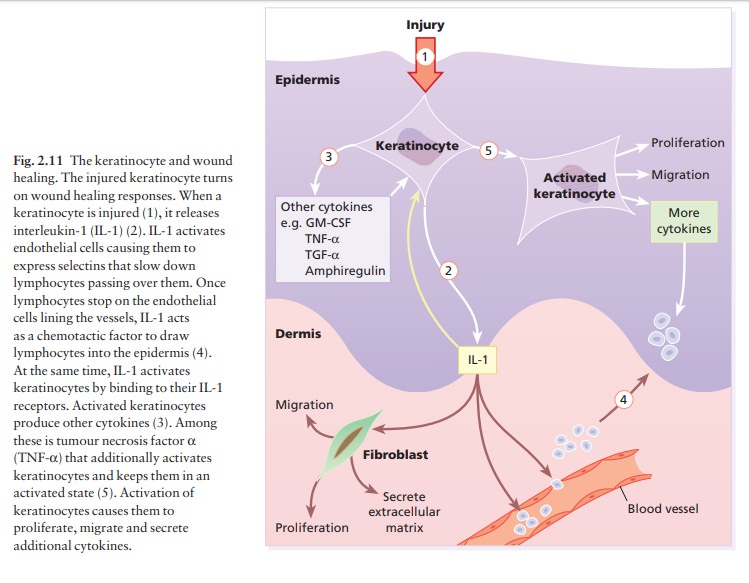
cytokines produced by injured keratinocytes (Fig. 2.11). For example,
keratinocyte-derived cytokines can activate Langerhans cells and T cells, and
IL-8, produced by keratinocytes, is a potent chemotactic factor for lymphocytes
and polymorphs, and brings these up into the epidermis.
Response to intracellular antigens
Antigens
coming from inside a cell, such as intra-cellular fungi or viruses and tumour
antigens, are presented to cytotoxic T cells (CD8+) by the
MHC Class I molecule. Presentation in this manner makes the infected cell
liable to destruction by cytotoxic T lymphocytes or K cells. NK cells can also
kill such cells, even though they have not been sensitized with antibody.
Granulomas
Granulomas
form when cell-mediated immunity fails to eliminate antigen. Foreign body
granulomas occur because material remains undigested. Immuno-logical granulomas
require the persistence of antigen, but the response is augmented by a
cell-mediated immune reaction. Lymphokines, released by lympho-cytes sensitized
to the antigen, cause macrophages to differentiate into epithelioid cells and
giant cells. These secrete other cytokines, which influence inflam-matory
events. Immunological granulomas of the skin are characterized by Langhans giant
cells (not to be confused with Langerhans cells;), epithelioid cells, and a
surrounding mantle of lymphocytes.
Granulomatous
reactions also occur when organ-isms cannot be destroyed (e.g. in tuberculosis,
leprosy, leishmaniasis), or when a chemical cannot be eliminated (e.g.
zirconium or beryllium). Similar reactions are seen in some persisting
inflammations of undetermined cause (e.g. rosacea, granuloma annulare,
sarcoidosis, and certain forms of panniculitis).
Related Topics