Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Rheumatic Disorders
Rheumatic Diseases
Rheumatic Diseases
Commonly
called arthritis (inflammation of a joint) and thought of as one condition, the
rheumatic diseases are actually more than 100 different types of disorders that
primarily affect skeletal muscles, bones, cartilage, ligaments, tendons, and
joints of males and females of all ages. Some disorders are more likely to
occur at a particular time of life or to affect one gender more than the other.
The onset of these conditions may be acute or insidious, with a course possibly
marked by periods of remission (a period when disease symptoms are reduced or
absent) and exacerbation (a period when symptoms occur or increase). Treatment
can be simple, aimed at localized relief, or it can be complex, directed
to-ward relieving systemic effects. Permanent changes may result from the
disease.
There are several approaches to the classification
of rheumatic diseases. One basic system is to classify disease as either
mono-articular (affects a single joint) or polyarticular (affects multiple
joints) and then to further classify it as either inflammatory or noninflammatory
(Klippel, 2001). Conditions that may secon-darily affect the musculoskeletal
structure are also included, emphasizing the diversity of the rheumatic
diseases.
Pathophysiology
Understanding
the normal anatomy and physiology of the di-arthrodial
or synovial joints is key to
understanding the patho-physiology of the rheumatic diseases. The function of
the synovial joints is movement. Each synovial joint has a given range of
motion, although range of motion of movable joints varies be-tween individuals.
In
a normal synovial joint, a smooth, nearly friction-free, re-silient surface for
movement is provided by articular cartilage,which covers the bone end of the
joint. Lining the inner surface of the fibrous capsule is the synovial
membrane, which secretes fluid into the space between the bone ends. The
synovial fluid functions as a shock absorber and a lubricant, allowing the
joint to move freely.
The
joint is the area most commonly affected by the inflam-mation and degeneration
seen in rheumatic diseases. Despite the diversity of rheumatic diseases, from
localized involvement of one joint to systemic, multisystem disorders, they all
involve some de-gree of inflammation and degeneration, which may occur
simul-taneously. Inflammation is manifested in the joints as synovitis. In
inflammatory rheumatic diseases, the primary process is in-flammation as a
result of the immune response. Degeneration oc-curs as a secondary process,
resulting from the effect of pannus
(proliferation of newly formed synovial tissue infiltrated with in-flammatory
cells). The inflammation is a result of altered im-mune function.
Conversely,
in degenerative rheumatic diseases, inflammation occurs as a secondary process.
This synovitis is usually milder, is more likely to be seen in advanced
disease, and represents a re-active process. The synovitis is thought to result
from mechani-cal irritation from cartilage matrix
products.
INFLAMMATION
Inflammation
involves a series of related steps. With the triggering event, the antigen stimulus activates monocytes
and T lympho-cytes (also called T cells). Next, the immunoglobulin antibodies
form immune complexes with antigens. Phagocytosis of the im-mune complexes is
initiated, generating an inflammatory reaction (joint effusion, pain, and
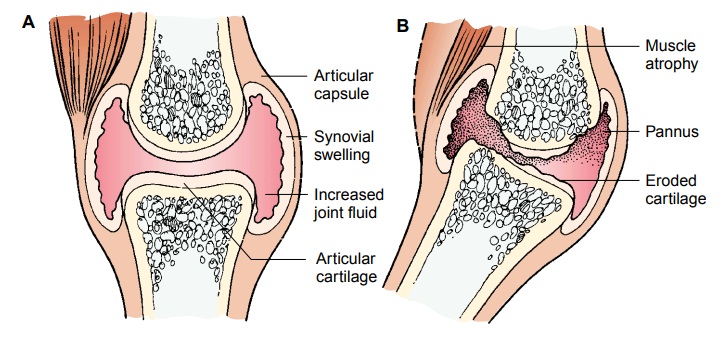
edema) (Fig. 54-1).
During
the next step, the normal immune response deviates. Phagocytosis produces
chemicals such as leukotrienes and prostaglandins. Leukotrienes contribute to the inflammatory process by attracting
other white blood cells to the area. Pros-taglandins
act as modifiers to inflammation. In some cases, theyincrease inflammation;
in other cases, they slow it down. Leuko-trienes and prostaglandins produce
enzymes such as collagenase that break down collagen, a vital part of a normal
joint. The re-lease of these enzymes in the joint causes edema, proliferation
of synovial membrane and pannus formation, destruction of carti-lage, and
erosion of bone.
The immunologic inflammatory process begins when antigens are presented to T lymphocytes, leading to a proliferation of T and B cells. B cells are a source for antibody-forming cells, or plasma cells. In response to specific antigens, plasma cells produce and release antibodies. Antibodies combine with corresponding antigens to form pairs, or immune complexes. The immune com-plexes build up and are deposited in synovial tissue or other or-gans in the body, triggering the inflammatory reaction that can ultimately damage the involved tissue.
The systemic nature of the rheumatic disease
category known as the diffuse connective tissue diseases is reflected in the
resul-tant widespread inflammatory process. Although focused in the joints,
inflammation also involves other areas. The blood vessels (vasculitis and arteritis),
lungs, heart, and kidneys may also be af-fected by the inflammation. In the
joints, this inflammatory re-sponse is manifested as pannus extending
throughout the joint space and, if persistent, eroding the articular cartilage,
causing secondary degenerative changes to the joint.
DEGENERATION
Although
the cause for degeneration of the articular cartilage is poorly understood, the
process is known to be metabolically active and therefore more accurately
called “degradation.” One theory of degradation is that genetic or hormonal
influences, mechanical factors, or prior joint damage causes cartilage failure.
Degrada-tion of cartilage ensues and increased mechanical stress on bone ends
causes stiffening of bone tissue. Another theory is that bone stiffening occurs
and results in increased mechanical stress on car-tilage, which in turn
initiates the processes of degradation.
Articular
cartilage plays two essential mechanical roles in joint physiology. First, the
articular cartilage provides a remarkably smooth weight-bearing surface and,
with synovial fluid, provides extremely low friction during movement. Second,
the cartilage transmits load or pressure to the bone, dissipating the
mechani-cal stress. Specific factors have been implicated in association with
degenerative joint changes.
Mechanical Stress.
Articular cartilage is highly resistant to wearunder conditions of repeated
movement. However, repetitive im-pact loading (velocity at which the force is
applied) rapidly leads to joint failure at the cartilage level. When a person
walks, three to four times the body weight is transmitted through the knee. A
deep knee bend transmits up to nine times the body weight through the
patellofemoral joint. As a joint undergoes repeated mechanical stress, the elasticity
of the joint capsule, articular car-tilage, and ligaments is reduced. The
articular plate (subchondralbone)
thins, and its ability to absorb shock decreases. The jointspace narrows,
accompanied by a loss of stability. When the ar-ticular plate disappears, bony
spurs (osteophytes) form at the
edges of the joint surfaces, and the capsule and synovial membranes
thicken. The joint cartilage degenerates and atrophies (shrinks), the bones
harden and hypertrophy (thicken) at their articular surfaces, and the ligaments
calcify. As a result, sterile joint
effusions (fluid escaping from the blood vessels or lym-phatics into the
joint cavity) and secondary synovitis may be pres-ent (Fig. 54-2).
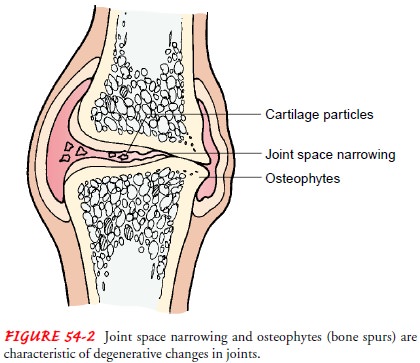
Altered Lubrication.
In addition to the changes in the articularcartilage and subchondral bone,
lubrication of the joint is also a factor in joint degeneration. With joint
loading (forces carried through the joint), lubrication depends on a film of
interstitial fluid squeezed out of the cartilage upon compression of the op-posing
surfaces of the joint. The mechanisms that normally op-erate under high weight
loads to produce this lubricating film may be affected.
Immobility.
Immobilization of a joint is another factor that canproduce degenerative changes in articular cartilage. Although these changes are more marked and appear earlier in areas of con-tact, they also occur in areas not subject to mechanical compres-sion. Cartilage degeneration due to joint immobility may result from loss of the pumping action of lubrication that occurs with joint movement. By 3 weeks after remobilization of the joint, the cartilage abnormalities are reversed. However, impact exercising (activities such as running) prevents reversal of the atrophy. In-stead, slow, gradual range of motion is thought to be very im-portant in preventing cartilage injury.
Clinical Manifestations
Pain
is the symptom of a rheumatic disease that most commonly causes a person to
seek medical attention. Other common symp-toms include joint swelling, limited
movement, stiffness, weak-ness, and fatigue.
Assessment and Diagnostic Findings
Assessment begins with a general health history,
which includes the onset of symptoms and how they evolved, family history, past
health history, and any other contributing factors. Because many of the
rheumatic diseases are chronic conditions, the health his-tory should also
include information about the patient’s perception of the problem, previous
treatments and their effectiveness, the patient’s support systems, and the
patient’s current knowledge base and the source of that information. A complete
health his-tory is followed by a complete physical assessment.
Assessment for rheumatic diseases combines the
physical ex-amination with a functional assessment. Inspection of the patient’s
general appearance occurs during initial contact. Gait, posture, and general
musculoskeletal size and structure are observed. Gross deformities and
abnormalities in movement are noted. The sym-metry, size, and contour of other
connective tissues, such as the skin and adipose tissue, are also noted and
recorded. Chart 54-1 outlines the important areas for consideration during the
physi-cal assessment. The functional assessment is a combination of his-tory
(what the patient reports that he or she can and cannot do) and examination
(observation of activities: the patient demon-strates what he or she can and
cannot do, such as dressing and getting in and out of a chair). Observation
also includes the adap-tations and adjustments the patient may have made
(sometimes without awareness); for example, with shoulder or elbow
involve-ment, the individual may bend over to reach the fork to the mouth
rather than raising the fork to the mouth.

The
history and physical assessment data are supplemented by supportive or
confirming diagnostic test findings. In some in-stances, tests are used to
follow the course of the disease. For example, the erythrocyte sedimentation
rate (ESR) reflects in-flammatory activity and indirectly the progression or remission
of disease. The following tests are most commonly used for pa-tients with
rheumatic diseases.
ARTHROCENTESIS
Arthrocentesis
(needle aspiration of synovial fluid) may be per-formed not only to obtain a
sample of synovial fluid for analysis but also to relieve pain caused by
pressure of increased fluid vol-ume, usually in the knee or shoulder. Synovial
fluid is usually an-alyzed in cases such as suspected joint infection to
determine the presence of inflammatory cells and to identify crystals in suspected
gout or the presence of blood following trauma.
After
the joint is anesthetized locally, a large-bore needle is in-serted into the
joint space to obtain a fluid specimen. Because this procedure has the
potential for introducing bacteria into the joint, aseptic technique is
essential. After the procedure, the pa-tient is observed for signs of infection
and hemarthrosis (bleed-ing into the
joint).
Normally, synovial fluid is clear, viscous,
straw-colored, and scanty in volume, with few cells. In inflammatory joint
disease, however, the fluid may become cloudy, milky, or dark yellow and may
contain numerous inflammatory cells, such as leukocytes (white blood cells) and
complement (a plasma protein
associated with immunologic reactions). The viscosity is reduced in
inflam-matory disease, and copious amounts of fluid may be present.
Arthrocentesis is diagnostically a valuable test, but arthrocentesis of small
joints (ie, fingers or wrist) to obtain fluid may be difficult.
X-RAY STUDIES
X-rays are often used in evaluating patients with
rheumatic dis-ease. The timing of the studies influences their usefulness: it
is un-likely that a patient with a 2-month history of joint inflammation will
have demonstrable changes on an x-ray study, but someone with long-standing
disease may show severe joint degeneration. X-rays can also be used to monitor
disease activity and progres-sion, demonstrating the loss of cartilage and
narrowing of the joint space over time. In addition, x-rays can demonstrate
carti-lage abnormalities, joint erosions, abnormal bony growth, and osteopenia
(decreased bone mineralization).
Arthrography.
Arthrography is a diagnostic procedure used todetect connective tissue
disorders. A radiopaque substance or air is injected into the joint cavity,
especially the knee or shoulder, to outline the contour of the joint. The joint
is then put through passive range of motion while several x-rays are obtained.
The ra-diopaque substance is absorbed systemically, and joint swelling
consequently subsides. After the procedure, the patient is ob-served for signs
of infection and hemarthrosis.
BONE AND JOINT SCANS
A bone scan reflects the degree to which the
crystal lattice of bone takes up or absorbs a bone-seeking radioactive isotope.
An area demonstrating increased uptake, such as a joint, is considered
ab-normal. A joint scan, the most sensitive study, allows determina-tion of
joint damage throughout the body. Because bone and joint scans are not the most
cost-effective methods for detecting early disease, they are not done routinely
at the time of diagnosis.
TISSUE BIOPSIES
A
muscle biopsy, carried out to examine skeletal muscle, is useful in diagnosing
myositis. Following administration of local anes-thesia under sterile
conditions in an outpatient setting or in an operating room, a surgical
incision is made to obtain the desired specimen, which is sent to the
laboratory for microscopic analysis. A pressure dressing is applied, and the
affected extremity is im-mobilized for 12 to 24 hours.
An arterial biopsy may be performed to examine a
specimen of an arterial wall using a procedure similar to that for a muscle
biopsy. Most frequently the temporal artery is selected, but other arteries may
be used as indicated. Arterial biopsy most often confirms in-flammation of the
vessel wall, or arteritis, a type of vasculitis.
A
skin biopsy may be performed to confirm inflammatory connective tissue diseases
such as lupus erythematosus or sclero-derma. A specimen may be lightly scraped
from the skin without causing discomfort. Deeper skin biopsies may be needed
when scraping is insufficient.
BLOOD TESTS
In general, laboratory studies in rheumatology are based on the assumption that most rheumatic diseases are autoimmune dis-orders.
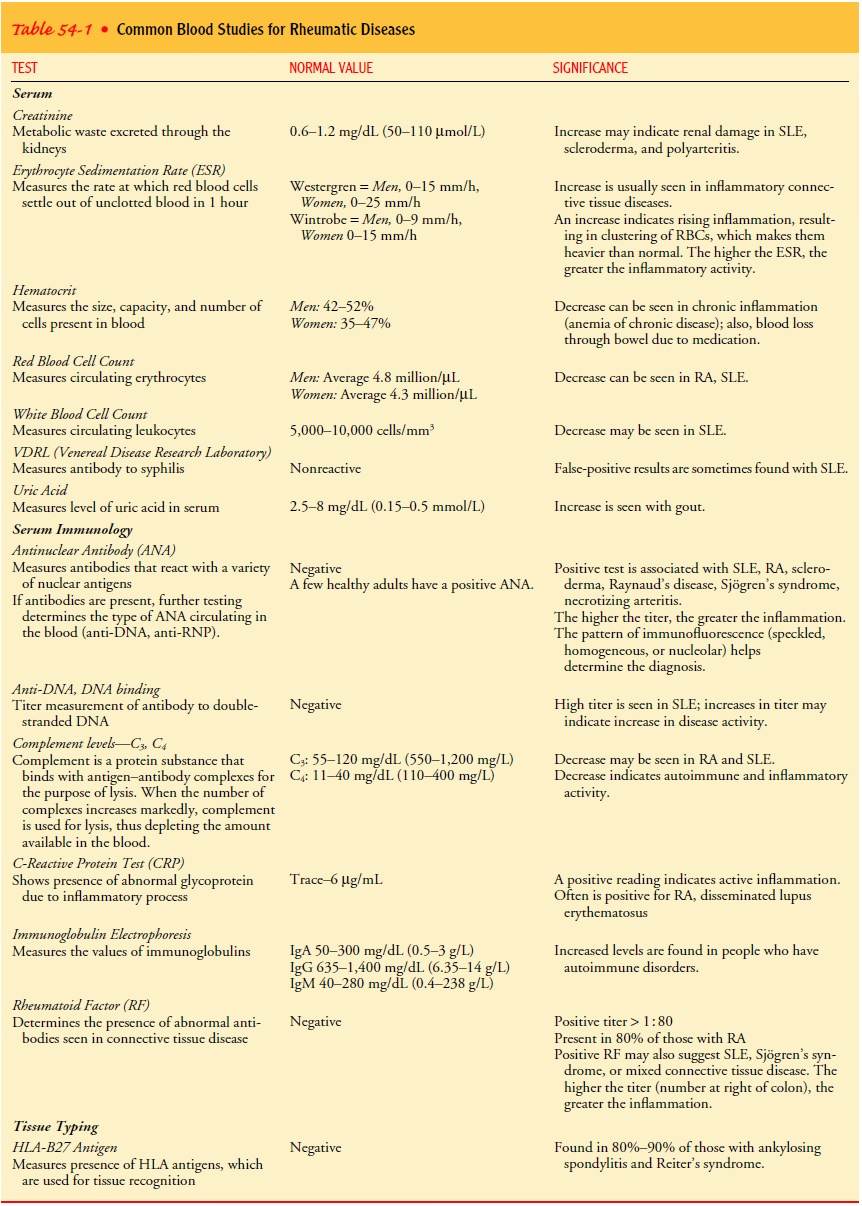
Although many of the tests are highly complex and technical,
no single test used in isolation sufficiently supports a diagnosis of a
rheumatic disease. Some of the most common blood studies are listed with their
corresponding normal ranges and primary indications in Table 54-1. Because many
of the tests require special laboratory techniques, they may not be used in
every health care facility. The physician determines which tests are necessary
based on the symptoms, stage of disease, and cost and likely benefit of the
test.
IMPLICATIONS
Diagnosis
of a specific rheumatic disease may or may not be rel-atively simple and
clear-cut. Commonly, observation of clinical signs and symptoms over time is
needed to make the diagnosis. The combination of history, assessment, testing,
and evolving manifestations of the disease may require explanation and
inter-pretation to the patient with early disease. This is especially true for
patients with multisystem rheumatic disease, such as one of the connective
tissue diseases.
The presence of crystals or bacteria in the
synovial fluid is specifically diagnostic for gout or infectious arthritis,
respectively. Diagnosis, however, may be more presumptive in the case of the
older person who is thought to have osteoarthrosis (OA) based on single joint
involvement, supportive x-ray findings, and no evidence of other disease
processes (Altman et al., 2000).
Many forms of rheumatic disease can be accurately
diagnosed by the primary health care provider, but patients with more
com-plicated signs and symptoms may need referral to a rheumatologist (a
physician who specializes in diagnosing and treating rheumatic disease).
Patients should know which type of rheumatic disease they have, not just that
they have “arthritis” or “arthritis of the knee.”
Gerontologic Considerations
Although
people of all ages, from infancy through childhood, adolescence, and maturity,
may be affected, rheumatic disease is commonly thought of by the patient,
family, and society as a whole as an inevitable consequence of aging. Many
older people expect and accept the immobility and self-care problems related to
the rheumatic diseases and do not seek help, thinking that nothing can be done.
Careful diagnosis and appropriate treat-ment can improve the quality of life
for older people. However, the rheumatic diseases do have some special
implications for the older adult.
In
elderly patients, other medical conditions may take prece-dence over the
rheumatic disease, which commonly becomes a secondary diagnosis and concern.
Identifying the effects of the rheumatic disease on the patient’s lifestyle,
independence, and other chronic or acute conditions is important.
The
frequency, pattern of onset, clinical features, severity, and effects on
function of the rheumatic disease in elderly patients may be different in very
elderly patients. Some of the rheumatic diseases, such as OA, are more
prevalent with aging (Altman et al., 2000). One disease, polymyalgia
rheumatica, is exclusive to the elderly (Gonzalez-Gay et al., 1999), whereas
some disorders may be less severe for elderly people than for younger patients.
How-ever, OA, the most prevalent activity-limiting condition among elderly
people, may account for more total disability among elderly patients than many
diseases, such as stroke or cancer, that are considered more serious.
In some instances, the age of the patient and
coexisting health problems may make diagnosis difficult. A missed diagnosis is
not unusual because of the assumption that most older people with joint
problems have OA. In addition, it may be difficult to dif-ferentiate problems
associated with aging from those caused by arheumatic disease. For example,
rheumatoid arthritis (RA) that begins in the later years has been shown to
differ prognostically and therapeutically from RA that begins in childhood or
early adulthood. In the elderly patient with initial RA, the onset is more
likely to be abrupt, but the clinical course does not appear to differ from
that of RA with an insidious onset. Moreover, pa-tients with elderly-onset RA
are less likely to have subcutaneous nodules or rheumatoid factor at disease
onset (Ruddy et al., 2001).
For the elderly person who has had a diffuse
connective tissue disease, the risk for osteoporosis is increased. Pain, loss
of mobil-ity, diminished self-image, and increasing morbidity can result from
progressive osteoporosis. Thus, diagnosis and treatment for osteoporosis should
not be overlooked in this population. Exer-cise, postural assistance, analgesic
agents, modification of activi-ties of daily living, and psychological support
can be useful.
Other conditions (eg, soft tissue problems such as
bursitis) usually are not problematic by themselves. When combined with other
health problems and the normal physiologic processes of aging, however, these
conditions may significantly reduce the patient’s quality of life. In fact, the
effects of most forms of rheumatic disease may lead to considerable changes in
the indi-vidual’s lifestyle, possibly threatening his or her independence. Decreased
vision and altered balance, often present in elderly peo-ple, may be
problematic if rheumatic disease in the lower extrem-ities affects locomotion.
Also, the combination of poor hearing, diminished vision, memory loss, and
depression contributes to nonadherence to the treatment regimen in elderly
patients. Special techniques for promoting patient safety, self-management, and
strategies such as memory aids for medications may be necessary.
Partly
because of the more frequent contact of the elderly with the health care system
for a variety of health issues, overtreatment or inappropriate treatment is
possible. Complaints of pain may be met with a prescription for an opioid
analgesic rather than in-structions for rest, use of an assistive device, and
local comfort measures such as heat or cold. Acetaminophen may be appropri-ate
and worth trying before using other medications that pose a greater chance of
side effects. Intra-articular corticosteroid injec-tions, with their usually
rapid relief of symptoms, may be re-quested by the patient who is unaware of
the consequences of too-frequent use. In addition to these factors, exercise
programs may not be instituted or may be ineffective because the patient
expects results to occur quickly or fails to appreciate the effec-tiveness of a
program of exercise.
Pharmacologic treatment of rheumatic disease in
older pa-tients is more difficult than it is in younger patients. If the
med-ications used have an effect on the senses (hearing, cognition), this
effect is intensified in the elderly. The cumulative effect of medications is
accentuated because of the physiologic changes of aging. For example, decreased
renal function in the elderly alters the metabolism of certain medications,
such as nonsteroidal anti-inflammatory drugs (NSAIDs). Elderly patients are
more prone to such side effects as gastroduodenal ulceration or bleeding, and
they are more likely to use nonprescription remedies, to try many different
medications (polypharmacy), and to be susceptible to unproven treatment methods
(Michocki, 2001).
Elderly patients with rheumatic disease may accept or endure pain, loss of ambulation, and difficulty with activities of daily living unnecessarily. The need to view oneself as capable of man-aging life independently despite increasing age may take consid-erable energy. The body image and self-esteem of the elderly person with rheumatic disease, combined with underlying depression, may interfere with the use of assistive devices such as canes. Use of adaptive equipment such as long-handled reachers or tongs may be viewed as evidence of aging rather than as a means of increasing independence.
The elderly person usually has a lifelong pattern
of dealing with the stresses of daily life. Depending on the success of that
pattern, the elderly person can often maintain a positive attitude and
self-esteem when faced with a rheumatic disease, especially when support is
available. Previous stress management strategies are assessed. If these
strategies have been effective, the patient is en-couraged and supported in
their use. If the strategies were ineffec-tive, the nurse assists the patient
in identifying alternative strategies, encourages use of new strategies, and
assesses their effectiveness.
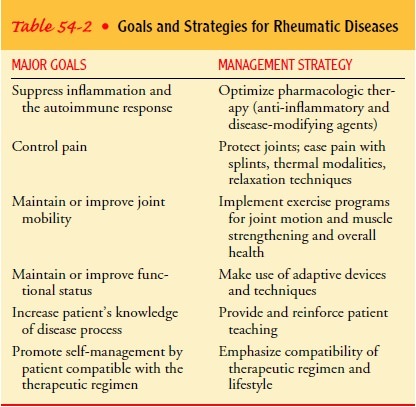
Medical Management
A
treatment program involving the interdisciplinary team, in-cluding the patient,
is the basis for managing the rheumatic dis-eases. The chronic nature of most
of these diseases mandates that the patient understand the disease, have the
information necessary to make good self-management decisions, and be presented
with a therapeutic program that is compatible with his or her lifestyle. Table
54-2 outlines the goals and strategies of basic rheumatic disease management.
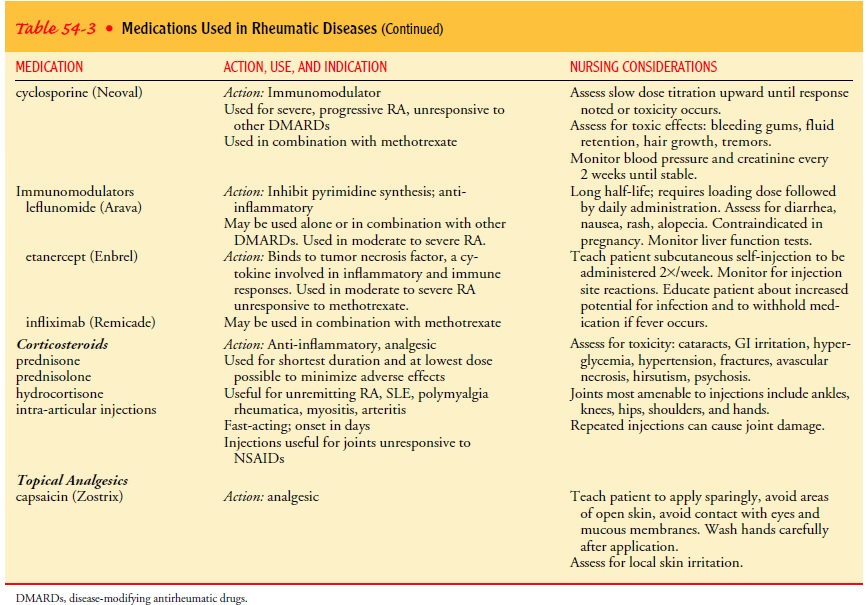
PHARMACOLOGIC THERAPY
Medications
are used with the rheumatic diseases to manage symptoms, control inflammation,
and in some instances to mod-ify the disease. Useful medications include the
salicylates, NSAIDs, and disease-modifying antirheumatic drugs. Table 54-3
reviews the medications often used.

Controlling
the inflammation related to the disease process will help in managing pain, but
this is often a delayed response. Nonopioid medications are often used for pain
management, especially early in the treatment program, until other measures can
be instituted (Burckhardt, 2001a). Short-term use of low-dose antidepressant
medications, such as amitriptyline, may be prescribed to reestablish adequate
sleep patterns and improve pain management (Wegner, 2001).
NONPHARMACOLOGIC PAIN MANAGEMENT
Nonpharmacologic methods of pain management are important. Methods used include therapeutic heat or cold and devices such as a cane or a wrist splint to protect and support the joint. A com-bination of methods may be required because different methods often work better at different times.
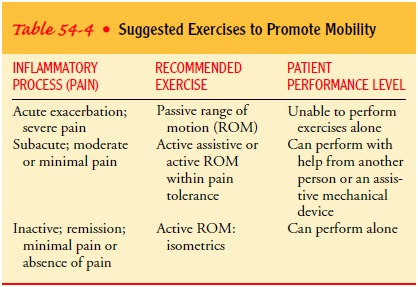
Exercise and Activity
The
ongoing nature of most rheumatic diseases makes it impor-tant to maintain and,
when possible, improve joint mobility and overall functional status. The individualized
exercise program is crucial to movement. Table 54-4 summarizes the exercises
ap-propriate for patients with rheumatic diseases. Appropriate pro-grams of
exercise have been shown to decrease pain and improve function. A mild
analgesic may be suggested prior to exercise for a patient starting a program
of exercise. Acute or prolonged pain associated with exercise should be
reported to a health care provider for evaluation (Minor & Westby, 2001).
The
major challenge for the patient and the health care provider is the need to
adjust all aspects of treatment according to the activity of the disease.
Especially for the patient with an active diffuse connective tissue disease,
such as RA or systemic lupus erythematosus (SLE), activity levels may vary from
day to day and even within the day itself.
Related Topics