Chapter: Mechanical : Engineering Thermodynamics : The Second Law of Thermodynamics
Reversible Process
Reversible Process
A process is said to be
reversible if it can be reversed without leaving any trace on the surroundings.
For
example, let a system be taken from state 1 to state 2 with a work transfer of
+5 kJ and heat transfer of -10
kJ. If the process is reversible, while taking the system from state 2 to state
1, the work transfer must be -5
kJ and heat transfer must be +10 kJ. So that, both the system and surroundings
are returned to their initial states at the end of the process 2 to 1.
Irreversibility
and Causes of Irreversibility
The factors that make a
process irreversible are known as irreversibilities. Various forms of
irreversibilities are listed below.
a) Friction :
Friction occurs at the interface of two bodies moving relative to each
other. It
is the
main cause of
irreversibility in many
processes. Energy spent
in
overcoming
friction is dissipated in the form of heat which can never be
restored.
b) Heat transfer: Once heat is transferred from a body at higher
temperature to a body at lower
temperature,
it can never be reversed without the aid of an external agency.
c) Unresisted expansion: Consider
a vessel with two chambers as given in the arrangement as shown in Fig.
4.6. If the members separating the gas from vacuum is removed, gas will expand
and occupy the entire space. Since the expansion has no influence on the
surroundings, there is no work output in this process. But to restore the
initial arrangement, a definite work input is required.
d) Mixing of two gases: Consider
a vessel with two chambers, one with O2 and the other with N2.
When the member separating O2 & N2 is removed,
uniform mixing is taking place without any work output. But such a process
cannot be reversed without any work input.
e) Throttling: It
is a totally irreversible process. Gas or vapour will expand through a restricted
passage with its pressure decreasing rapidly without any work output. Such an
expansion cannot be reversed.
Externally
and internally reversible processes
As mentioned earlier if
no irreversibilities occur outside the system boundaries during the process, it
is known as externally reversible.
If no irreversibilities
occur within the boundary of the system during a process, it is known as
internally reversible process. For such a process, the path of the reverse
process will follow exactly that of the forward process in any property
diagram.
To be totally
reversible or simply reversible both external and internal reversibilities must
be ensured.
The Carnot Cycle
In
1824, Nicholas Sadi Carnot proposed a classical ideal cycle consisting of four
processes. All processes are individually reversible and hence the cycle as a
whole is a reversible cycle. The processes that make up the Carnot cycle are :
Process 1-2
The
working substance is taken in a piston cylinder arrangement as given in Figure
4.8(a). Heat is added reversibly and isothermally from a high
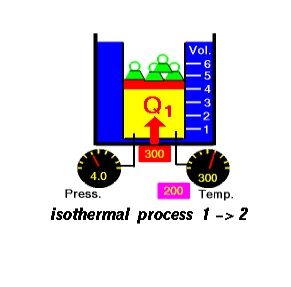
temperature
reservoir at TH. Since the process is to be reversible, the
temperature TH of the reservoir should be equal to or
infinitesimally greater than that of the working substance.
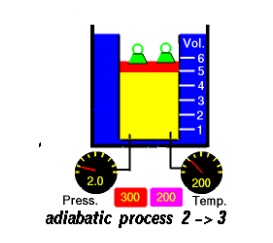
Process 2-3
The
working substance is allowed to expand reversibly and adiabatically until its
temperature falls down to TL. The process is represented by Figure
4.8(b)
Process 3-4
Heat
is rejected by the working substance to a low temperature reservoir kept TL
or at temperature infinitesimally smaller than TL.
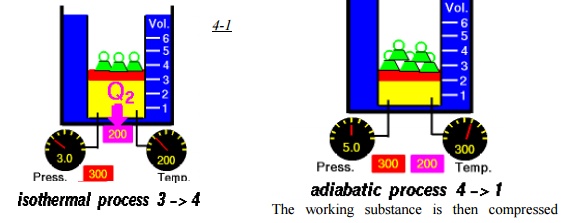
reversibly and
adiabatically until its temperature becomes TH and the cycle
continues.
The cycle has been
represented in a p-V diagram in Figure 4.9. The included area represents the
net work done in the cycle. From first law of thermodynamics net workdone is
equal to net heat transfer in the cycle. Since QH is the heat added
to system and QL is the heat rejected by the system, the neat heat
transfer
is QH -QL.
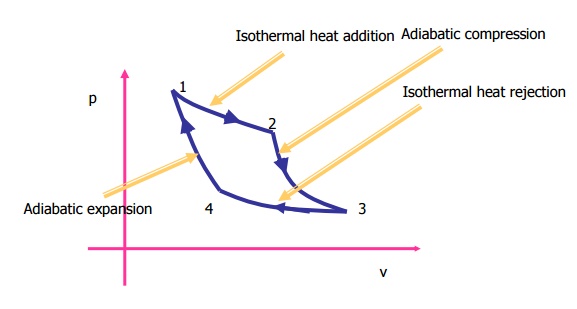
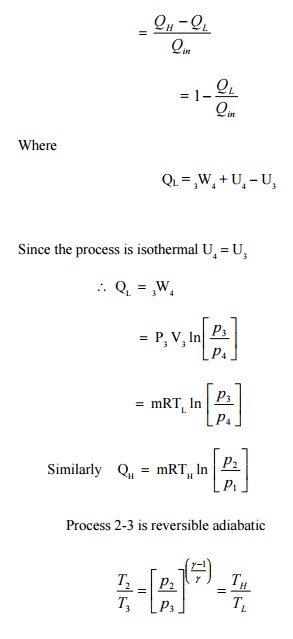
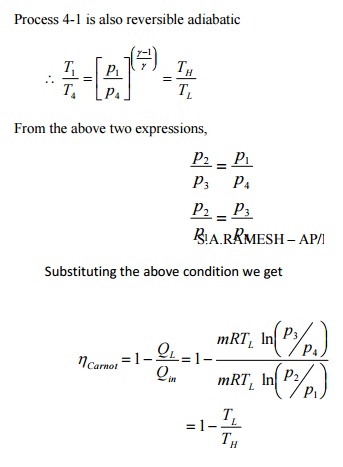
It
shows that efficiency of carnot engine is purely a function of TH
and TL.
Since the carnot cycle
being completely reversible, if carried out in reverse direction, the
magnitudes of all energy transfers remain the same but their sign change. This
reversed carnot cycle can be applied for a refrigerator or a heat pump. Figure
4.10 shows the p-V diagram of a reversed carnot cycle.
Related Topics