Chapter: Clinical Anesthesiology: Anesthetic Management: Obstetric Anesthesia
Regional Anesthetic Techniques: Local Anesthetic/Local Anesthetic Opioid Mixtures
Local
Anesthetic/Local Anesthetic–Opioid Mixtures
Epidural and spinal (intrathecal) analgesia
more commonly utilizes local anesthetics either alone or with opioids for labor
and delivery. Analgesia during the first stage of labor requires neural
blockade at the T10–L1 sensory level, whereas pain relief during the second
stage of labor requires neural blockade at T10–S4. Continuous lumbar epidural
analgesia is the most versatile andmost commonly-employed technique, because it
can be used for pain relief for the first stage of labor as well as
analgesia/anesthesia for subsequent vagi-nal delivery or cesarean section, if
necessary. “Single-shot” epidural, spinal, or combined spinal epidural
analgesia may be appropriate when pain relief is ini-tiated just prior to
vaginal delivery (the second stage). Obstetric caudal injections have largely
been abandoned because of less versatility; although effective for perineal
analgesia/anesthesia they require large volumes of local anesthetic to
anesthe-tize upper lumbar and lower thoracic dermatomes. They have also been
associated with early paralysis of the pelvic muscles that may interfere with
normal rotation of the fetal head, and with a small risk of accidental puncture
of the fetus.
Absolute contraindications to regional
anesthe-sia include patient refusal, infection over the injection site,
coagulopathy, marked hypovolemia, and true allergies to local anesthetics. The
patient’s inability to cooperate may prevent successful regional anes-thesia.
Neuraxial anesthesia and full anticoagulation is a dangerous combination.
Regional anesthesia should generally not be performed within 6–8 h of a
subcutaneous minidose of unfractionated heparin or within 12–24 h of
administration of low-molecu-lar-weight heparin (LMWH). Thrombocytopenia or
concomitant administration of an antiplatelet agent increases the risk of
spinal hematoma. A vaginal birth after cesarean (VBAC) delivery is not
consid-ered a contraindication to regional anesthesia dur-ing labor. Concern
that the anesthesia may mask pain associated with uterine rupture during VBAC
may not be justified, because dehiscence of a lower segment scar frequently
does not cause pain even without epidural anesthesia; moreover, changes in
uterine tone and contraction pattern may be more reliable signs.
Before performing any regional block,
appro-priate equipment and supplies for resuscitation should be checked and
made immediately available. Minimum supplies include oxygen, suction, a mask
with a positive-pressure device for ventilation, a functioning laryngoscope and
blades, endotracheal tubes (6 or 6.5 mm), oral and nasal airways, intrave-nous
fluids, ephedrine, atropine, propofol, and suc-cinylcholine. The ability to
frequently monitor blood pressure and heart rate is mandatory. A pulse
oxim-eter and capnograph should be readily available.
Lumbar Epidural Analgesia
Epidural analgesia for labor may be
administered in early labor after the patient has been evaluated by her obstetrician. When dilute mixtures of a local anesthetic and an
opioid are used, epidural analgesia has little if any effect on
the progress of labor. Concerns that regional analgesia will increase the
likelihood of oxytocin augmentation, operative (eg, forceps) delivery, or
cesarean section, are unjustified. It is often advantageous to place an
epidural catheter early, when the patient is less uncomfortable and can be
positioned more easily. Moreover, should an urgent or emergent cesarean section
become necessary, the presence of a well-functioning epidural catheter makes it
possible to avoid general anesthesia.
A. Technique
Parturients may be positioned on their sides
or in the sitting position for the procedure. The sitting position often makes
it easier to identify the midline and spine in obese patients. When epidural
anesthe-sia is being given for vaginal delivery (second stage), the sitting position
helps ensure good sacral spread.
Because the lumbar epidural space pressure
may be positive in some parturients, correct iden-tification of the epidural
space may be difficult. Unintentional dural puncture will occur even in
experienced hands; the incidence of “wet taps” in obstetric patients is
0.25–9%, depending on clinician
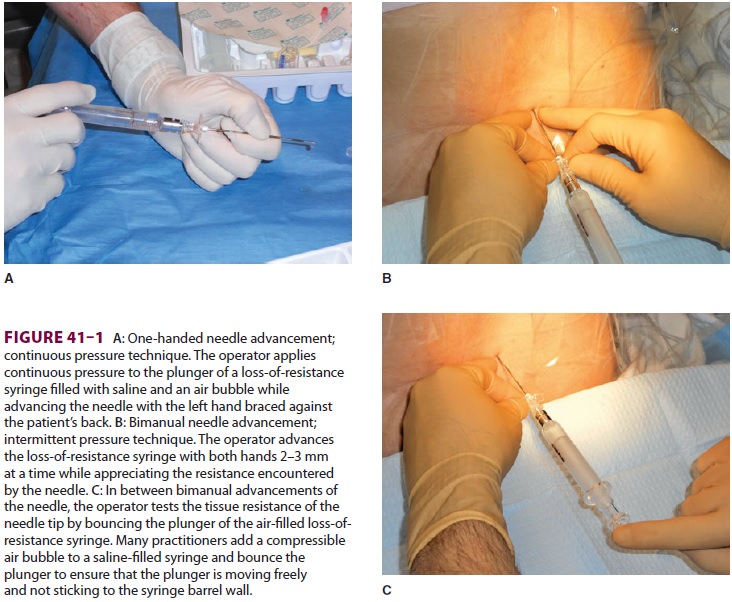
experience. Many practitioners add a
compress-ible air bubble to the saline syringe and bounce the plunger to ensure
that it moves freely and does not stick to the syringe wall ( Figure
41–1A and C).
Most clinicians advocate the midline approach, whereas a minority favors the
paramedian approach. For the placement of a lumbar epidural catheter in the
obstetric patient, most anesthesiologists advance the epidural needle with the
left hand, which is braced against the patient’s back, while applying
continuous pressure to a glass syringe filled with sterile saline (Figure
41–1A and C).
Alternatively, some make use of the “wings” of the Weiss epidural needle by
advancing it with both hands few millimeters at a time ( Figure
41–1B). A change of tissue resistance is then
tested continuously using tactile feedback when advancing the needle and by
intermittently applying pressure to the air-filled loss-of resistance syringe.
The later technique allows for precise con-trol of needle advancement and may
allow a better distinction of various tissue densities. If air is used for
detecting loss of resistance, the amount injected should be limited; injection
of larger volumes of air (>2–3 mL) in the epidural space has been associated with patchy or
unilateral analgesia and headache. The average depth of the lumbar epidural
space in obstetric patients is reported to be 5 cm from the skin. Placement of
the epidural catheter at the L3–4 or L4–5 interspace is generally optimal for
achiev-ing a T10–S5 neural blockade. Ultrasound guidance has recently been
offered as tool in assisting with the placement of an epidural catheter. This
technique allows the practitioner to judge the depth of the epi-dural space and
estimate the best angle of needle insertion. The potential benefit of this
technique is most obvious in obese patients with poor anatomic landmarks.
However, the technique is highly user-dependent, and few practitioners have
adopted it.
If unintentional dural puncture occurs, the anesthetist has two choices:
(1) place the epidural catheter in the subarachnoid space for continu-ous
spinal (intrathecal) analgesia and anesthesia , or (2) remove the needle and
attempt placement at a higher spinal level. The intrathecally-placed epidural
catheter may be used as continuous spinal anesthetic, possibly reducing the
incidence of post–dural puncture headache. If used in this fash-ion, an
infusion of 0.0625–0.125% bupivacaine with fentanyl, 2–3 mcg/mL starting at 1–3
mL/h, is a rea-sonable choice.
B. Choice of Epidural Catheter
Many clinicians advocate use of a multiholed
cath-eter instead of a single-holed catheter for obstetric anesthesia. Use of a
multiholed catheter may be associated with fewer unilateral blocks and greatly
reduces the incidence of false-negative aspiration when assessing for
intravascular or intrathecal cath-eter placement. Advancing a multiholed
catheter 4–6 cm into the epidural space appears to be optimal for obtaining
adequate sensory levels. A single-hole catheter need only be advanced 3–5 cm
into the epi-dural space. Shorter insertion depths (<5 cm), how-ever, may favor dislodgment of the
catheter out of the epidural space in obese patients following flexion/
extension movements of the spine. Spiral wire-reinforced catheters are very
resistant to kinking. A spiral or spring tip, particularly when used without a
stylet, is associated with fewer, less intense pares-thesias and may also be
associated with a lower inci-dence of accidental intravascular insertion.
C. Choice of Local Anesthetic Solutions
The addition of opioids to local anesthetic
solutions for epidural anesthesia has dramatically changed the practice of
obstetric anesthesia. The synergy between epidural opioids and local anesthetic
solu-tions reflects separate sites of action, namely, opiate receptors and
neuronal axons, respectively. When the two are combined, very low
concentrations of both local anesthetic and opioid can be used. More importantly,
the incidence of adverse side effects, such as hypotension and drug toxicity,
is likely reduced. Although local anesthetics can be used alone, there is
rarely a reason to do so. Moreover, when an opioid is omitted, the higher
concentration of local anesthetic required (eg, bupivacaine, 0.25%, and
ropivacaine, 0.2%) for adequate analgesia can impair the parturient’s ability
to push effectively as labor progresses. Bupivacaine or ropivacaine in
con-centrations of 0.0625–0.125% with either fentanyl, 2–3 mcg/mL, or
sufentanil, 0.3–0.5 mcg/mL, is most often used. In general, the lower the
concentration of the local anesthetic the greater the concentration of opioid
that is required. Very dilute local anes-thetic mixtures (0.0625%) generally do
not produce motor blockade and may allow some patients to ambulate (“walking”
or “mobile” epidural). The long duration of action of bupivacaine makes it a
popu-lar agent for labor. Ropivacaine may be preferable because of its reduced
potential for cardiotoxicity . At equi-analgesic doses, ropiva-caine and
bupivacaine appear to produce the same degree of motor block.
The effect of epinephrine-containing solutions on the course of labor is
somewhat controversial. Many clinicians use epinephrine-containing solu-tions
only for intravascular test doses because of concern that the solutions may
slow the progres-sion of labor or adversely affect the fetus; others use only
very dilute concentrations of epinephrine such as 1:800,000 or 1:400,000.
Studies comparing these various agents have failed to find any differences in
neonatal Apgar scores, acid–base status, or neurobe-havioral evaluations.
D. Epidural Activation for the First Stage of Labor
Initial epidural injections may be done
either before or after the catheter is placed. Administration through the
needle can facilitate catheter place-ment, whereas administration through the
catheter ensures proper function of the catheter. The follow-ing sequence is
suggested for epidural activation:
·
Test for unintentional subarachnoid
or intravascular placement of the needle or catheter with a 3-mL test dose of a
local anesthetic with 1:200,000 epinephrine (controversial; see the section on
Prevention of Unintentional Intravascular and Intrathecal Injections). Many
clinicians test with lidocaine 1.5% because of less toxicity following
unintentional intravascular injection and a more rapid onset of spinal
anesthesia than with bupivacaine and ropivacaine. The test dose should be
injected between contractions to help reduce false positive signs of an
intravascular injection (ie, tachycardia due to a painful contraction).
·
If after 5 min signs of
intravascular or intrathecal injection are absent, with the patient supine and
left uterine displacement, administer 10 mL of the local anesthetic– opioid
mixture in 5-mL increments, waiting 1–2 min between doses, to achieve a T10–L1
sensory level. The initial bolus is usually composed of 0.1–0.2% ropivacaine or
0.0625–0.125% bupivacaine combined with either 50–100 mcg of fentanyl or 10–20
mcg of sufentanil.
·
Monitor with frequent blood pressure
measurements for 20–30 min or until the patient is stable. Pulse oximetry
should also be used. Oxygen is administered via face mask if there are any
significant decreases in blood pressure or oxygen saturation readings.
·
Repeat steps 2 and 3
when pain recurs until the first stage of labor is completed. Alternatively, a
continuous epidural infusion technique may be employed using bupivacaine or
ropivacaine in concentrations of 0.0625–0.125% with either fentanyl, 1–5
mcg/mL, or sufentanil, 0.2–0.5 mcg/mL at a rate of 10 mL/h, which subsequently
is adjusted to the patient’s analgesic requirements (range: 5–15 mL/h). A third
choice would be to use patient-controlled epidural analgesia (PCEA). Some
studies suggest that total drug requirements may be less and patient
satisfaction is greater with PCEA compared with other epidural techniques. PCEA
settings are typically a 5-mL bolus dose with a 5–10 min lockout and 0–12 mL/h
basal rate; a 1-h limit of 15–25 mL may used. Migration of the epidural
catheter into a blood
vessel during a continuous infusion technique
may be heralded by loss of effective analgesia; a high index of suspicion is
required because overt signs of systemic toxicity may be absent. Erosion of the
catheter through the dura results in a slowly progressive motor blockade of the
lower extremities and a rising sensory level.
E. Epidural Administration During the Second Stage of Labor
Administration for the second stage of labor extends the block to
include the S2–4 dermatomes. Whether a catheter is already in place or epidural
anesthesia is just being initiated, the following steps should be undertaken:
·
If the patient does not already have
a catheter in place, identify the epidural space while the patient is in a
sitting position. A patient who already has an epidural catheter in place
should be placed in a semiupright or sitting position prior to injection.
·
Give a 3-mL test dose of local
anesthetic (eg, lidocaine 1.5%) with 1:200,000 epinephrine. Again, the
injection should be completed between contractions.
·
If aft er 5 min signs of an
intravascular or intrathecal injection are absent, give 10–15 mL of additional
local anesthetic–opioid mixture at a rate not faster than 5 mL every 1–2 min.
·
Administer oxygen by
face mask, lay the patient supine with left uterine displacement, and monitor
blood pressure every 1–2 min for the first 15 min, then every 5 min thereafter.
F. Prevention of Unintentional Intravascular and Intrathecal Injections
Safe administration of epidural anesthesia is
criti-cally dependent on avoiding unintentional intrathecal or intravascular injection. Unintentional intravascular or
intrathecal placement of an epidural needle or
catheter is possible even when aspiration fails to yield blood or cerebrospinal
fluid (CSF). The incidence of unintentional intravascular or intrathecal
placement of an epidural catheter is 5–15% and 0.5–2.5%, respectively. Even a
properly placed catheter can subsequently erode into an epidural vein or an intrathecal
position. This possi-bility should be considered each time local anes-thetic is
injected through an epidural catheter.
Test doses of lidocaine, 45–60 mg,
bupivacaine, 7.5–10 mg, ropivacaine, 6–8 mg, or chloroprocaine, 100 mg, can be
given to exclude unintentional intra-thecal placement. Signs of sensory and
motor block-ade usually become apparent within 2–3 min and 3–5 min,
respectively, if the injection is intrathecal.
In patients not receiving β-adrenergic antago-nists, the intravascular
injection of a local anesthetic solution with 15–20 mcg of epinephrine
consistently increases the heart rate by 20–30 beats/min within 30–60 s if the
catheter (or epidural needle) is intra-vascular. This technique is not always
reliable in parturients because they often have marked spon-taneous baseline
variations in heart rate with con-tractions. In fact, bradycardia has been
reported in a parturient following intravenous injection of 15 mcg of
epinephrine. Moreover, in animal studies, 15 mcg of epinephrine intravenously
reduces uterine blood flow. Alternative methods of detecting unintentional
intravascular catheter placement include eliciting tinnitus or perioral
numbness following a 100-mg test dose of lidocaine or eliciting a chronotropic
effect following injection of 5 mcg of isoproterenol. The use of dilute local
anesthetic solutions and slow injection rates of no more than 5 mL at a time
may also enhance detection of unintentional intravascular injections before
catastrophic complications develop.
G. Management of Complications
1. Hypotension—Generally defined as agreater than 20% decrease in the patient’sbaseline blood pressure, or a systolic blood pressure less than 100 mm Hg, hypotension is a common side effect of neuraxial anesthesia. It is primarily due to decreased sympathetic tone and is greatly accentu-ated by aortocaval compression and an upright or semiupright position. Treatment should be aggres-sive in obstetric patients and consists of intravenous boluses of ephedrine (5–15 mg) or phenylephrine (25–50 mcg), supplemental oxygen, left uterine dis-placement, and an intravenous fluid bolus. Although the routine use of a crystalloid fluid bolus prior to dosing an epidural catheter is not effective in the prevention of hypotension, ensuring proper intrave-nous hydration of the pregnant patient is important.
Use of the head-down (Trendelenburg) position
is controversial because of its potentially detrimental effects on pulmonary
gas exchange.
2. Unintentional intravascular injection—Earlyrecognition of intravascular injection, facilitated by the use of
small, repeated doses of local anesthetic instead of a large bolus, may prevent
more serious local anesthetic toxicity, such as seizures or cardio-vascular
collapse. Intravascular injections of toxic doses of lidocaine or
chloroprocaine usually pres-ent as seizures. Propofol, 20–50 mg, will terminate
seizure activity. Maintenance of a patent airway and adequate oxygenation are
critical; however, imme-diate endotracheal intubation with succinylcholine and
cricoid pressure is rarely necessary. Intravascular injections of bupivacaine
can cause rapid and pro-found cardiovascular collapse as well as seizure
activ-ity. Cardiac resuscitation may be exceedingly difficult and is aggravated
by acidosis and hypoxia. An imme-diate infusion of 20% Intralipid has shown
efficacy in reversing bupivacaine-induced cardiac toxicity. Amiodarone is the
agent of choice for treating local anesthetic–induced ventricular arrhythmias.
3.Unintentional intrathecal injection—Even whendural puncture is recognized immediately after injection of local anesthetic, attempted aspiration of the local anesthetic will usually be unsuccessful. The patient should be placed supine with left uter-ine displacement. Head elevation accentuates the adverse cerebral effects of hypotension and should be avoided. Hypotension should be treated with phen-ylephrine and intravenous fluids. A high spinal level can also result in diaphragmatic paralysis, which necessitates intubation and ventilation with 100% oxygen. Delayed onset of a very high and often patchy or unilateral block may be due to unrecognized sub-dural injection , which is managed similarly.
4.Postdural puncture headache (PDPH)—Headache frequently follows unintentional dural puncture in parturients. A self-limited headache may occur without dural puncture; in such instances, injection of significant amounts of air into the epi-dural space during a loss-of-resistance technique may be responsible. PDPH is due to decreased intra-cranial pressure with compensatory cerebral vaso-dilation . Bed rest, hydration, oral analgesics, and caffeine sodium benzoate (500 mg added to 1000 mL intravenous fluids administered at 200 mL/h) may be effective in patients with mild headaches and as temporary treatment. Patients with moderate to severe headaches usually require an epidural blood patch (10–20 mL) . Prophylactic epidural blood patches are not rec-ommended; 25–50% of patients may not require a blood patch following dural puncture. Delaying a blood patch for 24 h increases its efficacy. Intracra-nial subdural hematoma has been reported as a rare complication 1–6 weeks following unintentional dural puncture in obstetric patients.
5. Maternal fever—Maternal fever is often inter-preted as chorioamnionitis and may trigger an invasive evaluation for neonatal sepsis. There is no evidence that epidural anesthesia affects maternal temperature or that neonatal sepsis is increased with epidural analgesia. An elevation in maternal tem-perature is associated with a high body mass index and with nulliparity in women and prolonged labor.
Combined Spinal & Epidural (CSE) Analgesia
Techniques using CSE analgesia and
anes-thesia may particularly benefit patients withsevere pain early in labor
and those who receive anal-gesia/anesthesia just prior to delivery. Intrathecal
opioid and local anesthetic are injected after which an epidural catheter is
left in place. The intrathecal drugs provide nearly immediate pain control and
have minimal effects on the early progress of labor, whereas the epidural
catheter provides a route for subsequent analgesia for labor and delivery or
anes-thesia for cesarean section. Addition of small doses of local anesthetic
agents to intrathecal opioid injec-tion greatly potentiates their analgesia and
can sig-nificantly reduce opioid requirements. Thus, many clinicians will
inject 2.5 mg of preservative-free bupivacaine or 3–4 mg of ropivacaine with
intrathe-cal opioids for analgesia in the first stage of labor. Intrathecal
doses for CSE are fentanyl, 5–10 mcg, or sufentanil, 5 mcg. Some studies
suggest that CSE techniques may be associated with greater patient satisfaction
and lower incidence of PDPH than epi-dural analgesia alone. A 24- to 27-gauge
pencil-point spinal needle (Whitacre, Sprotte, or Gertie Marx) is used to
minimize the incidence of PDPH.
The spinal and epidural needles may be placed
at separate interspaces, but most clinicians use a needle-through-needle
technique at the same inter-space. Use of saline for identification of the
epidural space may potentially cause confusion of saline for CSF. With the
needle-through-needle technique, the epidural needle is placed in the epidural
space and a long spinal needle is then introduced through it and advanced
farther into the subarachnoid space. A distinct pop is felt as the needle
penetrates the dura. The needle-beside-needle technique typically employs a
specially designed epidural needle that has a channel for the spinal needle.
After the intrathecal injection and withdrawal of the spinal needle, the epidural
catheter is threaded into position and the epidural needle is withdrawn. The
risk of advancing the epidural catheter through the dural hole created by the
spinal needle appears to be negligible when25-gauge or smaller needle is used.
The epidural catheter, however, should be aspirated carefully and local
anesthetic should always be given slowly and in small increments to avoid
unintentional intrathe-cal injections. Moreover, epidural drugs should be
titrated carefully because the dural hole may facili-tate entry of epidural
drugs into CSF and enhance their effects.
Spinal Anesthesia
Spinal anesthesia given just prior to delivery—also known as saddle
block—provides profound anesthe-sia for operative vaginal delivery. Use of a
22-gauge or smaller, pencil-point spinal needle (Whitacre, Sprotte, or Gertie
Marx) decreases the likelihood of PDPH. Hyperbaric tetracaine (3–4 mg),
bupiva-caine (2.5–5 mg), or lidocaine (20–40 mg) usually provides excellent
perineal anesthesia. Addition of fentanyl (12.5–25 mcg) or sufentanil (5–7.5
mcg) significantly potentiates the block. A T10 sensory level can be obtained
with slightly larger amounts of local anesthetic. Three minutes after
injection, the patient is placed in the lithotomy position with left uterine
displacement.
Related Topics