Chapter: Clinical Anesthesiology: Anesthetic Management: Obstetric Anesthesia
Anesthesia for Hypertensive Disorders
HYPERTENSIVE DISORDERS
Hypertension during pregnancy can be classified as pregnancy-induced
hypertension (PIH, often also referred to as preeclampsia), chronic
hypertension that preceded pregnancy, or chronic hypertension with superimposed
preeclampsia. Preeclampsia is usually defined as a systolic
blood pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm
Hg after the 20th week of ges-tation, accompanied by proteinuria (>300 mg/d)
and resolving within 48 h after delivery. When sei-zures occur, the syndrome is
termed eclampsia. The HELLP syndrome describes preeclampsia associated with hemolysis, elevated liver enzymes,
and a lowplatelet count. In the United States, preeclampsiacomplicates
approximately 7–10% of pregnancies; eclampsia is much less common, occurring in
one of 10,000–15,000 pregnancies. Severe preeclamp-sia causes or contributes to
20–40% of maternal deaths and 20% of perinatal deaths. Maternal deaths are
usually due to stroke, pulmonary edema, and hepatic necrosis or rupture.
Pathophysiology & Manifestations
The pathophysiology of preeclampsia is
probably related to a vascular dysfunction of the placenta that results in
abnormal prostaglandin metabolism. Patients with preeclampsia have elevated
production of thromboxane A2 (TXA2)
and decreased produc-tion of prostacyclin (PGI2). TXA2
is a potent vaso-constrictor and promoter of platelet aggregation, whereas PGI2 is a potent vasodilator and inhibitor of
platelet aggregation. Endothelial dysfunction may reduce production of nitric
oxide and increase pro-duction of endothelin-1. The latter is also a potent
vasoconstrictor and activator of platelets. Marked vascular reactivity and
endothelial injury reduce placental perfusion and can lead to widespread
sys-temic manifestations.
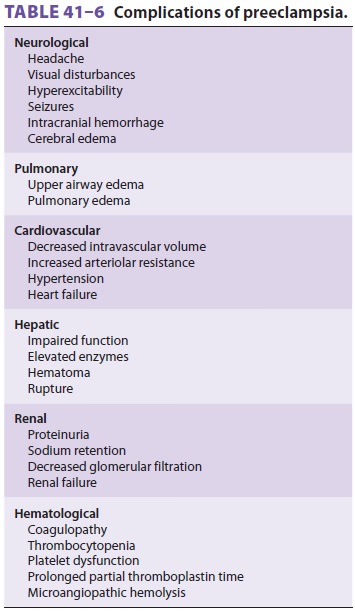
Severe preeclampsia substantially increases
both maternal and fetal morbidity and mortal-ity, and is defined by a blood
pressure greater than 160/110 mm Hg, proteinuria in excess of 5 g/d, oli-guria
(<500 mL/d), elevated
serum creatinine, intra-uterine growth restriction, pulmonary edema, central
nervous system manifestations (headache, visual dis-turbances, seizures, or
stroke), hepatic tenderness, or the HELLP syndrome (Table 41-6). Hepatic rupture may also occur in patients with the HELLP syndrome.
Patients with severe preeclampsia or
eclampsia have widely differing hemodynamic profiles. Most patients have
low-normal cardiac filling pressures with high systemic vascular resistance,
but cardiac output may be low, normal, or high.
Treatment
Treatment of preeclampsia consists of bed rest, seda-tion, repeated doses of antihypertensive drugs (usually labetalol, 5–10 mg, or hydralazine, 5 mg intrave-nously), and magnesium sulfate (4 g intravenous loading, followed by 1–3 g/h) to treat hyperreflexia and prevent convulsions. Therapeutic magnesium levels are 4–6 mEq/L.
Invasive arterial and central venous monitor-ing are indicated in
patients with severe hyperten-sion, pulmonary edema, or refractory oliguria; an
intravenous vasodilator infusion may be necessary. Definitive treatment of
preeclampsia is delivery of the fetus and placenta.
Anesthetic Management
Patients with mild preeclampsia generally
require only extra caution during anesthesia; standard anes-thetic practices
may be used. Spinal and epidural anesthesia are associated with similar
decreases in arterial blood pressure in these patients. Patients with severe
disease, however, are critically ill and require stabilization prior to
administration of any anesthetic. Hypertension should be controlled and
hypovolemia corrected before administration of anesthesia. In the absence of
coagulopathy, continuous epidural anes-thesia is the first choice for most
patients with pre-eclampsia during labor, vaginal delivery, and cesarean
section. Moreover, continuous epidural anesthesia avoids the increased risk of
a failed intubation due to severe edema of the upper airway.
A platelet count and coagulation profile
should be checked prior to the institution of regional anes-thesia in patients
with severe preeclampsia. It has been recommended that regional anesthesia be
avoided if the platelet count is less than 100,000/μL, but a platelet count as low as 70,000/μL may be acceptable in
selected cases, particularly when the count has been stable. Although some
patients have a qualitative platelet defect, the usefulness of a bleeding time
determination is questionable. Continuous epidural anesthesia has been shown to
decrease catecholamine secretion and improve uteroplacental perfusion up to 75%
in these patients, provided hypotension is avoided. Judicious fluid boluses
with epidural activation may be required to correct the disease-related
hypovolemia. Goal-directed hemodynamic and fluid therapy utiliz-ing arterial
pulse wave contour analysis (Virgileo/ Flotrac, LiDCOrapid) or echocardiography
may be employed to guide fluid replacement. Use of an epi-nephrine-containing
test dose for epidural anesthe-sia is controversial because of questionable
reliability (see earlier section Prevention of Unintentional Intravascular and
Intrathecal Injection) and the risk of exacerbating hypertension. Hypotension
should be treated with small doses of vasopressors because patients tend to be
very sensitive to these agents. Recent evidence suggests that spinal anesthesia
does not, as previously thought, result in a more severe reduction of maternal
blood pressure. Therefore, this technique is a reasonable anesthetic choice for
cesarean section in a preeclamptic patient.
Intraarterial blood pressure monitoring is
indi-cated in patients with severe hypertension during both general and
regional anesthesia. Intravenous vasodilator infusions may be necessary to
control blood pressure during general anesthesia. Intravenous labetalol (5–10
mg increments) can also be effective in controlling the hypertensive response
to intuba-tion and does not appear to alter placental blood flow. Because
magnesium potentiates muscle relaxants, doses of nondepolarizing muscle
relaxants should be reduced in patients receiving magnesium therapy and should
be guided by a peripheral nerve stimu-lator. The patient with suspected
magnesium toxic-ity, manifested by hyporef lexia, excessive sedation, blurred
vision, respiratory compromise and cardiac depression, can be treated with
intravenous admin-istration of calcium gluconate (1 g over 10 minutes).
Related Topics