Chapter: Mechanical : Heat and Mass Transfer : Phase Change Heat Transfer and Heat Exchangers
Regimes of Pool Boiling and Flow Boiling
REGIMES OF POOL BOILING AND FLOW BOILING
;
Regimes of Boiling
Let us consider a heating
surface (a wire or a flat plate) submerged In a pool of water which is at its
saturation temperature. If the temperature of the heated surface exceeds the
temperature of the liquid, heat energy will be transferred from the solid
surface to the liquid. From Newton's law of cooling, we have
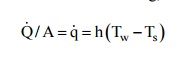
where
Q /A is the heat flux, Tw is the temperature of the heated surface
and Ts, is the temperature of the liquid, and the boiling process will start.
(i)
Pool Boiling - Pool boiling occurs only when the temperature of the heated
surface exceeds the saturation temperature of the liquid. The liquid above the
hot surface I s quiescent and its motion n ear the surface is due to free
convection.
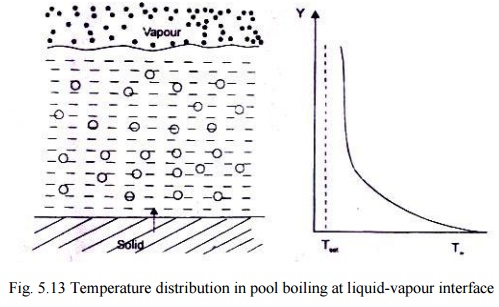
Fig.
5.13 Temperature distribution in pool boiling at liquid-vapour interface
Bubbles
grow at the heated surface, get detached and move upward toward the free
surface due to buoyancy effect. If the temperature of the liquid is lower than
the saturation temperature, the process is called 'subcooled or local boiling'.
If the temperature of the liquid is equal to the saturation temperature, the
process is known as 'saturated or bulk boiling'. The temperature distribution
in saturated pool boiling is shown in Fig5.13. When Tw exceeds Ts
by a few degrees, the convection currents circulate in the superheated liquid
and the evaporation takes place at the free surface of the liquid.
(ii)
Nucleate Boiling - Fig. I 1.5 illustrates the different regimes of boiling
where the heat flux Q / Ais plotted against the temperature difference (Tw -Ts
)When the temperature Tw increases a
little more, vapour bubbles are formed at a number of favoured spots on the
heating surface. The vapour bubbles are initially small and condense before
they reach the free surface. When the temperature is raised further, their
number increases and they grow bigger and finally rise to the free surface.
This phenomenon is called 'nucleate boiling'. It can be seen from the figure
(5.14) that in nucleate boiling regime, the heat flux increases rapidly with
increasing surface temperature. In the latter part of the nucleate boiling,
(regime 3), heat transfer by evaporation is more important and predominating.
The point A on the curve represents 'critical heat flux'.
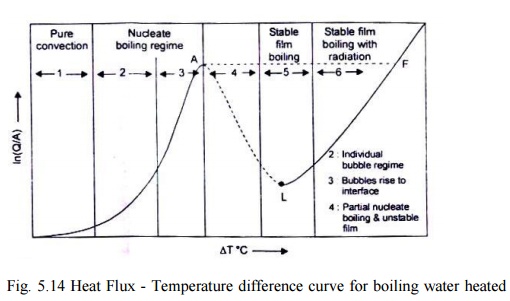
Fig. 5.14 Heat Flux -
Temperature difference curve for boiling water heated by a wire (Nukiyama's
boiling curve for saturated water at atmospheric pressure)
(L
is the Laidenfrost Point)
(iii)
Film Bolling –
point
A, a vapour film forms and covers the entire heating surface. The heat transfer
takes place through the vapour which is a poor conductor and this increased
thermal resistance causes a drop in the heat flux. This phase is film boiling'.
The transition from the nucleate boiling regime to the film boiling regime is
not a sharp one and the vapour film under the action of circulating currents
collapses and rapidly reforms. In regime 5, the film is stable and the heat
flow rate is the lowest.
(iv) Critical Heat Flux
and Burnout Point - For T beyond 550oC
(region 6) the temperature of the heating metallic surface is very high and the
heat transfer occurs predominantly by radiation, thereby, increasing the heat
flux. And finally, a point is reached at which the heating surface melts -
point F in Fig. 11.5. It can be observed from the boiling curve that the whole
boiling process remains in the unstable state between A and F. Any increase in
the heat flux beyond point A will cause a departure from the boiling curve and
there would be a large increase in surface temperature.
Related Topics