Matter Around Us | Term 1 Unit 3 | 6th Science - Pure Substances and Mixtures | 6th Science : Term 1 Unit 3 : Matter Around Us
Chapter: 6th Science : Term 1 Unit 3 : Matter Around Us
Pure Substances and Mixtures
Pure Substances and Mixtures
In shops, we find products which are sold as 100% pure! For common people pure means unadulterated- that which does not contain any cheap or harmful additives. Are these really pure substances as they claim to be?
For a Chemist the
word ‘pure’ means something else!
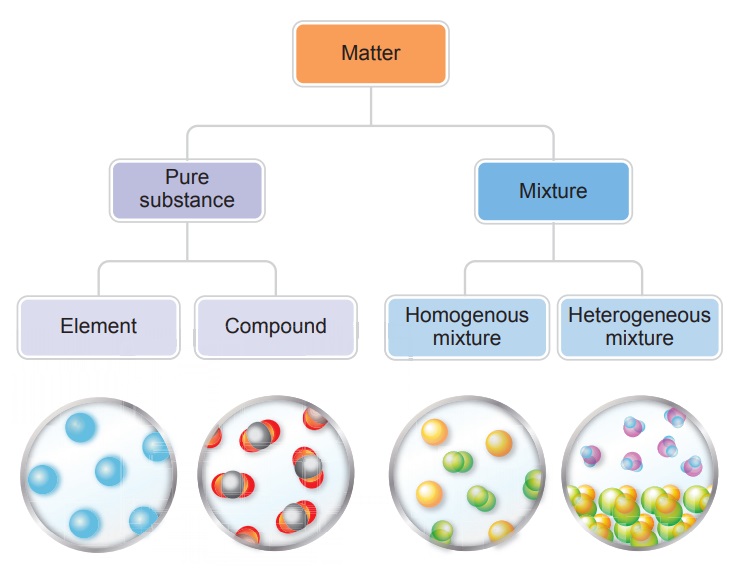
* A pure substance is made up of only
one kind of particles.
* Pure substances may be elements or
compounds.
* An atom is the smallest particle
that an element is made up of same kind of atoms. Molecule is the combination
of two or more atom. Compound in the substance formed by the chemical
combination of two or more element.

Purity of gold is expressed in terms
of ‘carat’. 24 carat gold is considered to be gold in its purest form.
Is matter around us
pure?
Fast facts
Let
us consider the following examples:

We have had the snack. Can you
identify and mention a few things that are present in snacks like - Mixture or
fruit mixture? You are able to identify the ingredients in them from their
colours, appearance or taste.
We mix rice, dal salt, chillies,
pepper, ghee and other ingredients to make Pongal. Pongal is also a mixture.
Why do we call these as mixtures- because
they are made up of more two or more ingredients or components that are
physically separable?
Explore
Can we always see
the different components of the mixture with our naked eyes?
Let us see the two
pictures given below:


In fig: we can see
and physically separate the components of vegetable salad, where as in fig: we
can neither see nor physically separate the components of an aerated drink-
soda water.
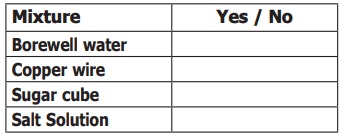
Try it yourself: Try to identify from the list what you
think may be classified as mixture. Write yes if it is a mixture, then write
No, if it is not a mixture. You may also write “I do not know” and later
discuss with your teacher.
Air is a mixture
because it contains Oxygen, Nitrogen, Carbon dioxide, water vapour, noble gases
etc.
Milk is also a
mixture of water, fat, protein etc.
Lemon juice is a
mixture. Some of us like it less sweet i.e. with less sugar; while some others
like it very sweet so they prefer to add more sugar. But either way, it is still
lemon juice-prepared from lemon extract, water and sugar and is a mixture
though the amount of sugar added is different. Same way even if we add extra
water or lemons extract it will still be a mixture. A mixture need not have a
fixed proportion of components.
* A Mixture is an impure substance and
contains more than one kind of particles.
* In the mixture the components are
mixed in any proportion.
When elements chemically combine they
form compounds; whereas a mixture can be a physical combination of
a. two or more elements. Example: 22
carat gold which is composed of gold and copper / gold and cadmium,
b. two or more compounds. Example:
aerated drink which is composed of carbon-di-oxide,
water, sweetening and colouring agents,
c. an element and compound. Example :
Tincture of iodine which is composed of Iodine in alcohol.
Related Topics