Term 1 Unit 3 | 6th Science - Matter Around Us | 6th Science : Term 1 Unit 3 : Matter Around Us
Chapter: 6th Science : Term 1 Unit 3 : Matter Around Us
Matter Around Us
Unit 3
Matter Around Us


Learning Objectives
At
the end of the lesson you will be able to
* Define
matter and develop an understanding of the particulate nature of matter
* Sort
the objects on the basis of certain properties
* Differentiate
between solids, liquids and gases based on the arrangement of their particles.
* Differentiate
between pure substances and mixtures
* Identify
the need for separation of mixtures
* Suggest
suitable methods for separating given samples of mixture
* Acquire an awareness on food adulteration and its harmful
effects
Introduction
Matter is all
around us. The air you are breathing is also a matter. Matter is defined as
anything that has mass and takes up space. Matter is found in three major states;
solid, liquid and gas. So what is matter made of? All matter is made of atoms.
Atoms are the smallest particle of matter.
They are so small
that you cannot see them with your eyes or even with a standard microscope. A standard
sheet of paper is about millions atoms thick. Science has come up with a
technology to identify structure of atoms Scanning Electron Microscope (SEM)
and Tunnelling Electron Microscope (TEM) which uses electricity to map atoms.
There is more about atoms in the later, but first let's learn about the three
states of matter. Silicon atoms on a surface via Scanning Tunneling
Microscopy, (STM).
Activity
– 1
Take a few crystals of sugar.
Observe them carefully with the help of a magnifying lens.
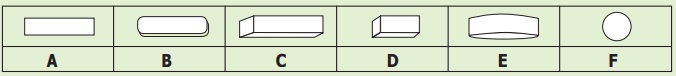
Which of the shapes given above
resembles a sugar crystal?
A B C D E F
Answer:
D
Now place a few sugar crystals in a
teaspoon full of water.
Answer: Like everything else a sugar crystal is also made up of molecules. When sugar dissolves in water, the sugar crystals break down and the molecules of sugar get distributed in the water. This makes the water taste sweet. The sugar molecules are extremely small, that is why we are not able to see them. A small amount of any matter will have many million molecules. (1 million = 1000000)
Like everything else a sugar crystal
is also made up of molecules. When sugar dissolves in water, the sugar crystals
break down and the molecules of sugar get distributed in the water. This makes
the water taste sweet. The sugar molecules are extremely small, that is why we
are not able to see them. A small amount of any matter will have many million
molecules. (1 million = 1000000)
Besides solids, Liquid and gases
there are two more states plasma and Bose – Einstein condensates.
Plasma is not a common state of
matter on Earth, but may be the most common state of matter in the universe.
For example, stars including sun are covered in plasma.
Bose – Einstein condensate is a gas
– like state of matter that exists at extremely cold temperatures. It was
predicted around 1925 and confirmed in 1995, This is used in the field of
cryogenics.
Related Topics