Chapter: 11th Biochemistry : Chapter 5 : Carbohydrates
Properties of Glucose, Fructose and Galactose
Properties of Glucose, Fructose and Galactose:
1. Glucose:
· Can be solid or liquid
· Melting Point: 294.8˚F(146˚C)
· Density: 1.54 g/cm³
· Weight: 180.16 g/mol
· Soluble in water and acetic acid
2. Fructose:
Fructose
has a higher solubility than other sugar; therefore, it is harder for fructose
to crystallize from an aqueous solution.
· White colour Powder
· Melting point of fructose is 103°C
· The compound of fructose has a molar mass of 180.16 mol/g.
· Density of 1.69g/cm2.
· Weight 180.16 g/mol
· Soluble in water
3. Galactose
· White powder.
· Solubility in water : 680 g/L.
· Melting point 167 °C
· Weight 180.156 g/mol.
· Soluble in water.
Chemical Properties:
Reactions of Glucose, Fructose and Galactose:
i. Acidic character:
Both glucose and fructose behave as
weak acids and form salts with Ca(OH)2 (lime water).
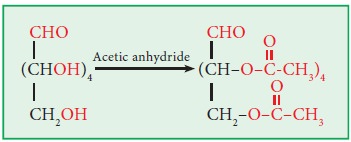
ii. Ester formation:
Glucose and Fructose form penta
acetyl derivative when treated with acetic anhydride.
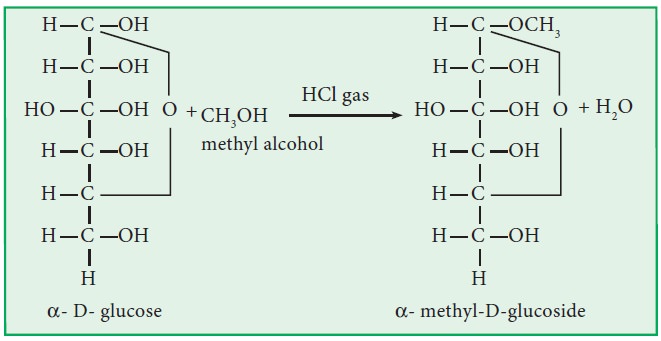
iii. Ether formation:
Glucose and fructose react with
methanol in the presence of dry HCl gas to give ethers known as methyl
glucoside and methyl fructoside, respectively.
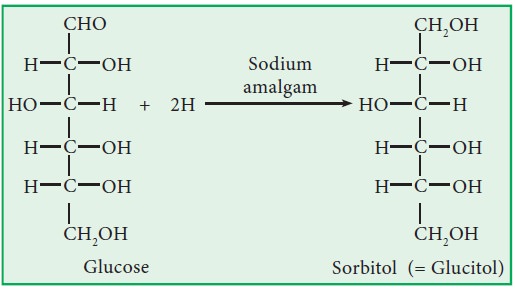
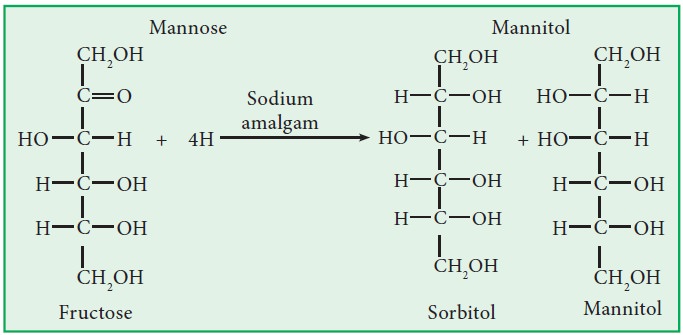
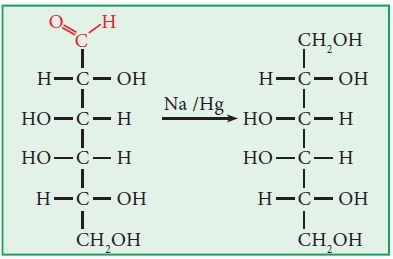
iv. Reduction:
i.
Sodium amalgum
reduces glucose into sorbitol and fructose into a mixture of sorbitol and
mannitol. Similarly, it reduces fructose into a mixture of sorbitol and
mannitol.
a.Both
are reduced to n-hexane by HI / red ‘P’.
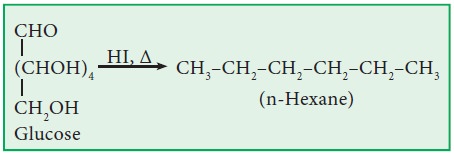
b.Galactose
on reduction with Na/Hg, gives dulcitol ( and with HI/red P, n-hexane will be
obtained.
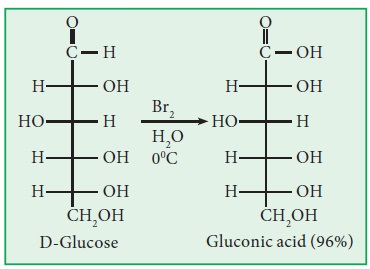
v. Oxidation:
a. Glucose
is oxidized by mild oxidizing agents like bromine water into gluconic acid.
Strong oxidizing agents like conc. HNO3 oxidize glucose into
gluconic acid.
b. Fructose
is not oxidised by mild oxidising agents. But strong oxidising agents like
conc. HNO3 split fructose into a mixture of trihydroxy glutaric,
tartaric and glycollic acids.
c. Galactose
on oxidation with mild oxidizing agent such as bromine water, gives galactonic
acid. On oxidation with strong oxidizing agents like HNO3, it gives
galactaric (or) mucic acid. This acid is insoluble in water and hence this
reaction is used as a test for galactose. On oxidation with O2/Pt-C
(as in glucose, after protecting the –CHO group into ispropylidene group) it
gives galacturonic acid.
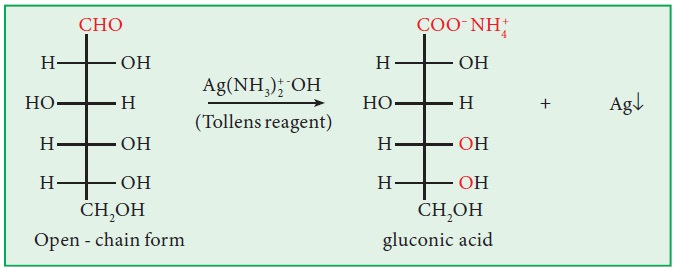
vi. Action with Tollen’s reagent:
Both
glucose and fructose reduce Tollen’s reagent into silver mirror.
vii. Action with Fehling’s solution:
Both
glucose and fructose reduce Fehling’s solution into red cuprous oxide.
viii. Action with Barfoed’s and Benedict’s reagents:
Both
glucose and fructose reduce Barfoed’s and Benedict’s reagents into red cuprous
oxide as in the case of Fehling’s solution.
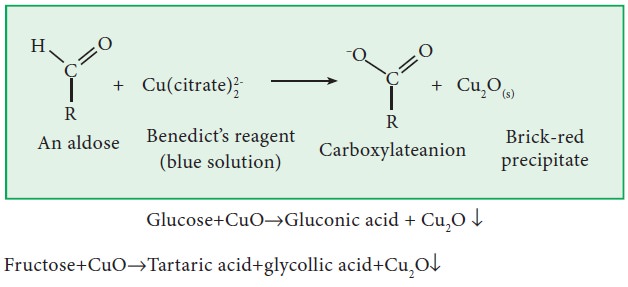
Glucose+CuO‚Gluconic acid + Cu2O ↓
Fructose+CuO‚Tartaric acid+glycollic acid+Cu2O↓
Since
both glucose and fructose reduce all these four reagents (Tollen’s, Fehling’s,
Benedict’s and Barfoed’s reagent), these sugars are known as reducing sugars.
ix. Action with hydroxylamine:
· Both glucose and fructose form oximes with
hydroxylamine.
· With NH2OH, galactose forms
galactoseoxime.
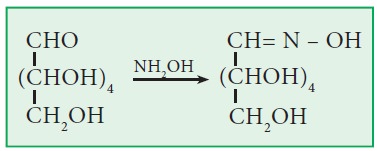
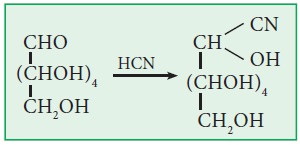
x. Action with HCN (Kiliani synthesis):
Both
glucose and fructose form cyanohydrins with HCN
xi. Action with Conc. HCl:
Both
glucose and fructose when heated with conc. HCl give laevulic acid.
xii. Action with alkalies:
When
warmed with conc. alkali sugars first turn yellow, then brown and finally
resinify. But in the presence of dilute. alkali glucose and fructose give a
mixture of D-glucose, D-mannose and D-fructose. This is known as Lobry de Bruyn
- van Ekenstein rearrangement. This occurs through enediol.
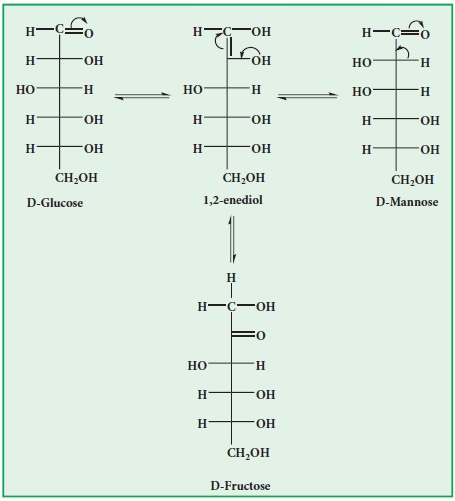
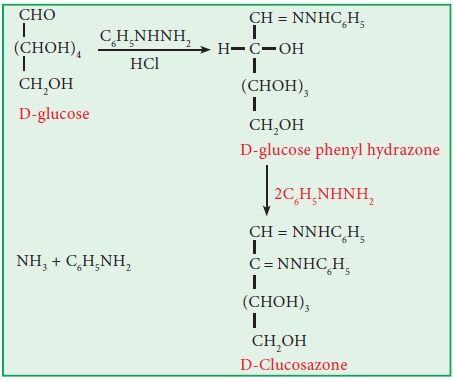
xiii. Osazone formation:
Both
glucose and fructose react with excess of phenyl hydrazine to give same type of
osazone. Glucose and fructose have structural difference with respect to only
first two carbon atoms, which are involved in osazone formation. The
configurations in the rest of the carbon atoms are similar to both glucose and
fructose. Hence they form similar osazone.
Related Topics