Example, Source, Structure, Uses | Carbohydrates - Polysaccharides | 11th Biochemistry : Chapter 5 : Carbohydrates
Chapter: 11th Biochemistry : Chapter 5 : Carbohydrates
Polysaccharides
POLYSACCHARIDES
Carbohydrates which contain more than 10 monosaccharide units
are known as polysaccharides. Example : Starch, cellulose, glycogen, inulin
etc.
1. HOMOPOLYSACCHARIDES
Starch:
a) Source:
Plant
materials such as roots, tubers, stem, vegetables, fruits and cereals are the
main sources of starch.
b) Structure:
Starch
is the nutritional reservoir in plants. Starch is a homopolysaccharide consists
of only α-D-glucose. The two chief constituents of starch are (i) Amylose (15-20%)
and (ii) Amylopectin (80 - 85%).
Amylose
forms the inner portion of the starch grain and is soluble in water. It is linear,
non-branched polymer of glucose. The glucose residues are united by α(1-4)
linkage. The molecular weight of amylose is 60,000.
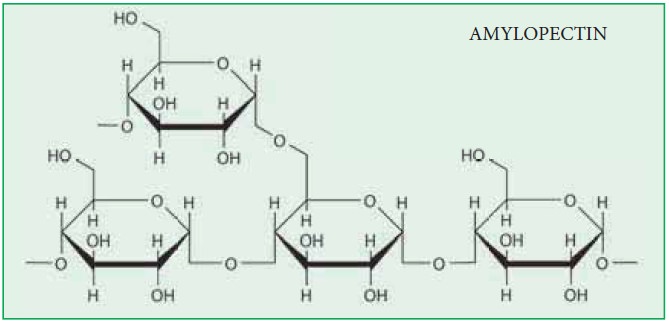
Amylopectin
forms the outer covering of the starch grain and is insoluble in water. It is a
highly branched polymer of glucose. The glucose residues are united by α(1- 4)
linkages in the chains and by α (1 - 6) at the branch points. Its molecular
weight is 2,00,000. It is like glycogen except its lower degree of branching.
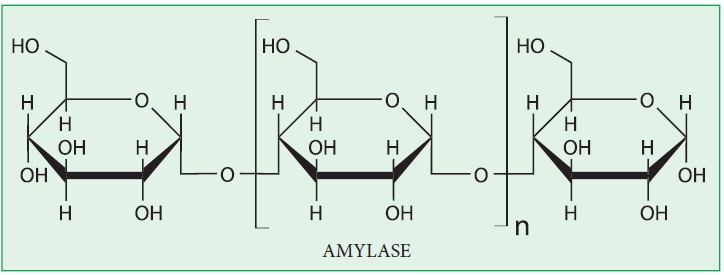
c) Hydrolysis of starch :
Starch
is hydrolysed both by acids and enzymes. Both amylose and amylopectin are
rapidly hydrolysed by α-amylase which is secreted by salivary glands and
pancreas. α-Amylase acts upon starch and hydrolyses it into finally maltose
molecules.
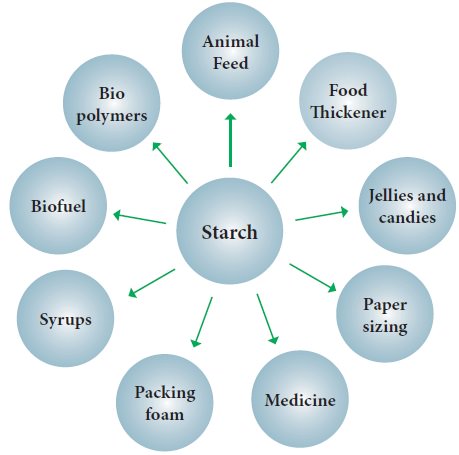
Uses : Starch is used as
(i) as a
food material.
(ii)for
the manufacture of glucose and alcohol.
(iii) in
paper industry.
(iv) in textile industry.
(v) in printing. to prepare starch acetate, nitrostarch
etc.
(vii)
for making adhesives.
(viii) as
an indicator.
Glycogen :
a) Source :
Glycogen is the carbohydrate reserve
in animals; hence often referred as animal starch. It is present in the high
concentration in liver, muscle and brain.
b) Structure :
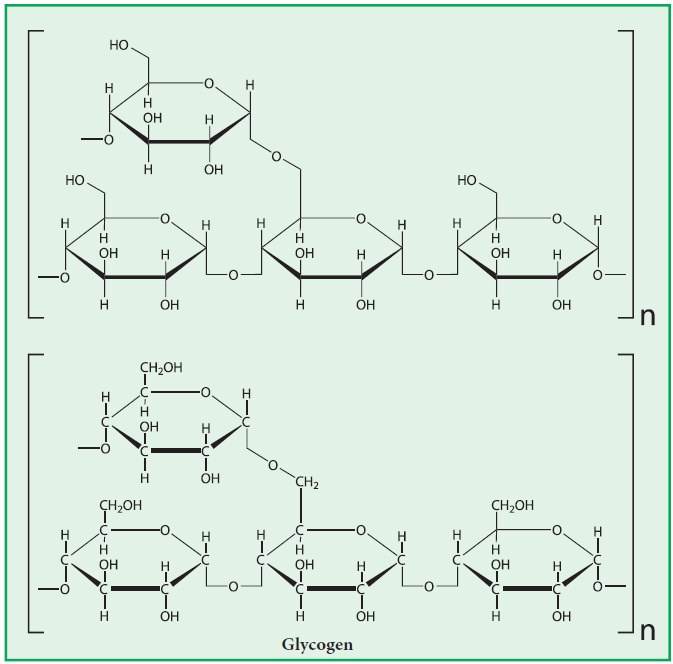
Glycogen
is very large, branched polymer of glucose residues. The structure of glycogen
is similar to that of amylopectin with more number of branches. The glucose
units in glycogen are linked by α(1-4) glycosidic bonds and α-(1-6) glycosidic
bonds at branching points. Branching occurs about once in 10 units. The
molecular weight (upto 1 × 108) and the number of glucose units
(upto 25,000) vary in glycogen depending upon the source.
Uses :
·
Excess carbohydrate
in the body are deposited as glycogen.
· Animal glycogen is used as food.
2. HETEROPOLYSACCHARIDES (HETEROGLYCANS)
Glycosaminoglycans :
· Glycosaminoglycans are otherwise known as mucopolysaccharides.
· These are heteroglycans made up of repeating unit
of aminosugars and uronic acids.
· Because of the presence of charged groups (carboxyl
group, sulphate group, acetylated amino group), they attract water molecules
and so they produce viscous solutions.
· Some of the mucopolysaccharides are found in
combination with protein to form mucoproteins (or) mucoids (or) proteoglycans.
They contain 95% carbohydrate and 5% protein.
· Examples : i) hyaluronic acid ii) heparin iii)
chondroitin sulpahte iv) keratan sulphate v) dermatan sulphate.
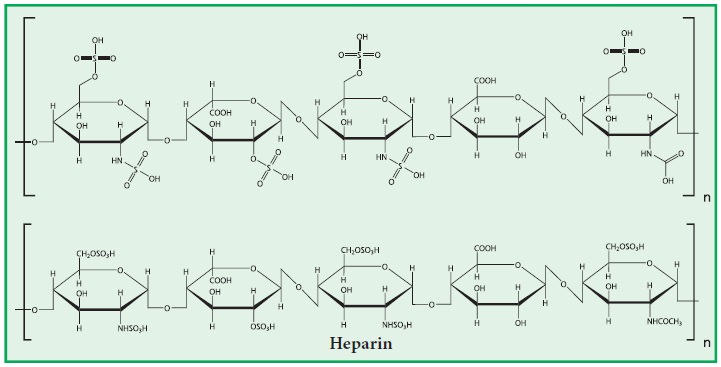
(i) Heparin:
·
It is a mucopolysaccharide present in liver, lung, spleen, kidney, and
blood.
·
It is a blood
anticoagulant.
·
Heparin is composed
of alternating units of N-sulpho-glucosamine-6 sulphate and L-iduronate-2-sulphate.
·
These two molecules
are held together by α(1-4) glycosidic bond.
·
Its molecular
weight 20,000.
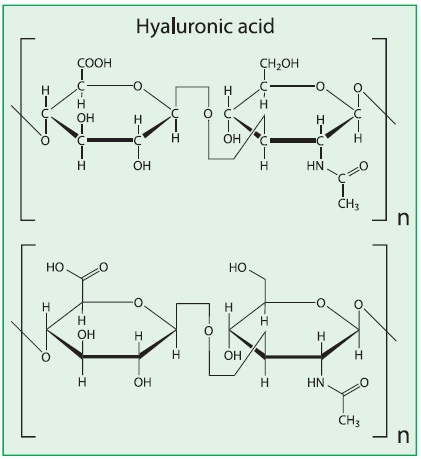
ii) Hyaluronic acid :
·
It is a
mucopolysaccharide present in syanovial fluid, vitreous humor of eyes,
cartilage tissues, loose connective tissues and in bacteria.
·
It consists of
repeated units of α-glucuronic acid and N-acetylglucosamine.
·
These two molecules
are held together by α(1-3) glycosidic bond.
·
It is an unbranched
chain polymer.
·
Its solutions are
viscous and hence acts as lubricant and shock absorbent in joints.
·
In tissues, it acts
as a barrier and permits the metabolites to pass through but not the bacteria
and other infectious agents.
·
Hyaluronic acid
contains about 250-25,000 disaccharide units, held by α-(1-4) glycosidic bonds
with a molecular weight upto 4 million.
· The α(1-4) linkage in hyaluronic acid is cleaved by the enzyme
hyaluronidase. This enzyme is present in high concentration in testes, seminal
fluid and certain snake venom.
Related Topics