Example, Properties, Structure | Carbohydrates - Disaccharides | 11th Biochemistry : Chapter 5 : Carbohydrates
Chapter: 11th Biochemistry : Chapter 5 : Carbohydrates
Disaccharides
Disaccharides
The carbohydrates which on hydrolysis
give two monosaccharide units are known as disaccharides.
Example: Sucrose, maltose,
lactose etc.,
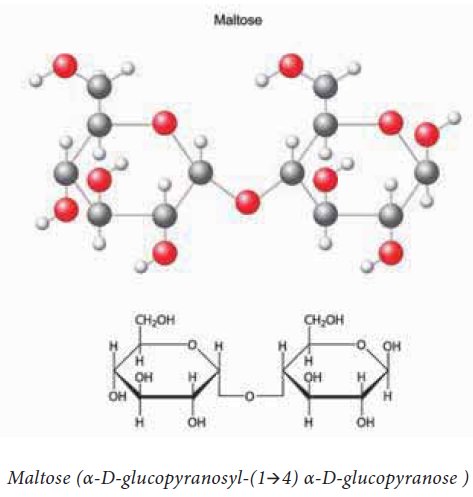
1. Maltose:
Properties of Maltose:
a. Maltose or malt sugar is formed as an intermediate product in
the acid hydrolysis of starch.
b. It is also produced during the course of digestion of starch by
pancreatic amyalse.
c.
It is a reducing
disaccharide.
d. Maltose is composed of two α-D-glucose units held together by
α(1-4) glycosidic linkage.
e.
It is hydrolyzed by
dilute acids (or) enzyme maltase into two α - D - glucose units.
f.
Maltose is readily
fermented by yeast.
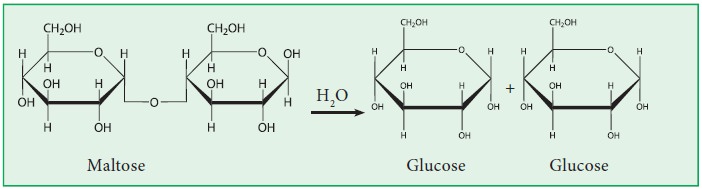
Structure of Maltose
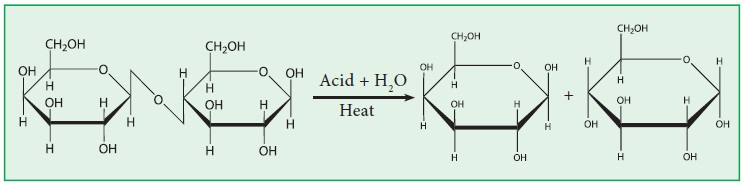
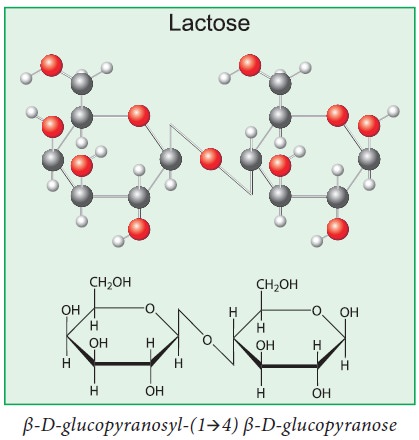
2. Lactose :
a. Lactose is formed by the mammary glands. It is milk
sugar.
b. It is a reducing sugar, forms osazone.
c. It is hydrolysed by acids and enzyme lactase into
one molecule of α- D- galactose and one molecule of α - D - glucose.
d. It is fermented by yeast.
e. In the lactose, the galactose and glucose units are
held together by α(1-4) linkage.
Structure of Lactose:
3. Sucrose:
Properties of sucrose:
· When heated to 200°C it loses water to form a brown
mass called caramel. On strong heating, it gives pure carbon with a burnt
smell.
· Concentrated sulphuric acid dehydrates sucrose into
carbon. This on further oxidation by H2SO4 gives CO2
C12H22O11+H2SO4‚-->
C + H2O +SO2
· When boiled with HCl, sucrose gives laevulic acid.
· Concentrated nitric acid oxidises cane sugar
(sucrose) to oxalic acid.
C12H22O11+9O2
--> 6(COOH)2+ 5H2O
· Sucrose is fermented by invertase into glucose and
fructose which are converted to ethanol by zymase. Both these enzymes are
available in yeast.
· Sucrose on acetylation gives octa-acetyl
derivative.
· Sucrose on methylation gives octa-o-methyl
derivative.
· Sucrose does not react with HCN, NH2OH,
phenyl hydrazine, Tollen’s reagent and Fehling’s solution.
· Controlled reduction of sucrose gives a mixture of
sorbitol and mannitol.
· It reacts with lime water Ca(OH)2 to
give calcium sucrate.
Hydrolysis of sucrose:
Sucrose
is hydrolysed by dilute acids or enzymes like sucrase or invertase into an
equimolar mixture of glucose and fructose.
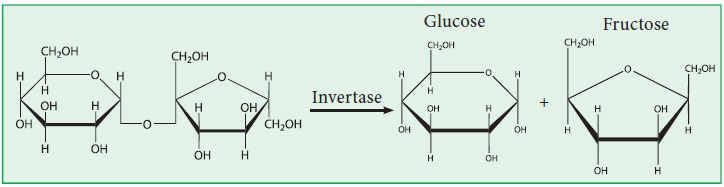
Sucrose is dextrorotatory. But the
hydrolysed product is laevorotatory. Since the direction of rotation is
reversed, this phenomenon is known as inversion of cane sugar. The mixture of
sugars formed on hydrolysis is known as invert sugar.
According to Hudson, sucrose is first
split into α-D(+) glucopyranose and β-D(+) fructofuranose, both are dextro
rotatory. However, the less stable β-D(+) fructofuranose then sets up an
equilibirum with its more stable isomer, α-D(–) fructopyranose which is
strongly laevorotatory. Thus, the invert sugar gives a specific rotation of
–28.2°.
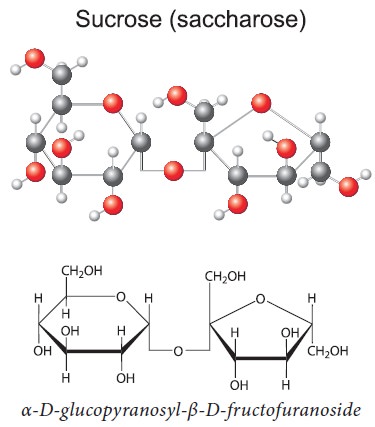
Structure of Sucrose:
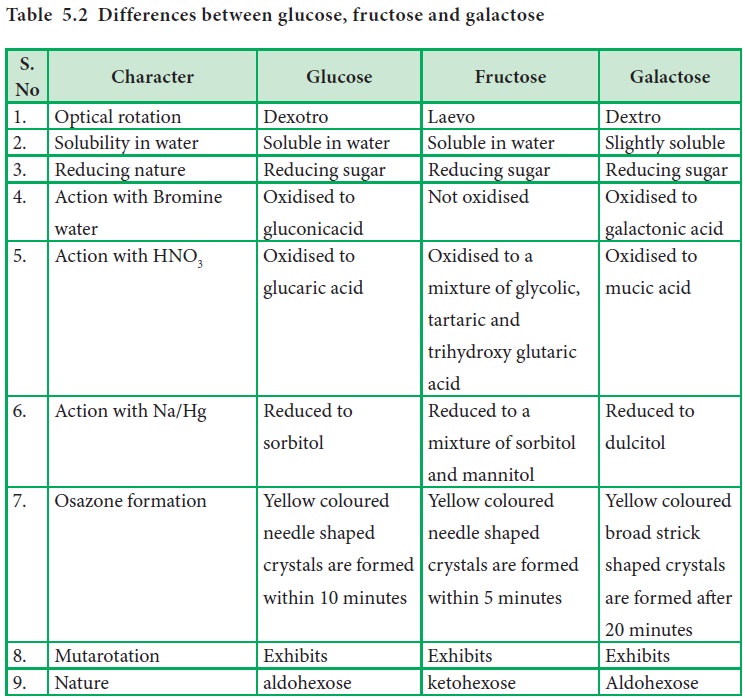
Table
5.2 Differences between glucose, fructose and galactose
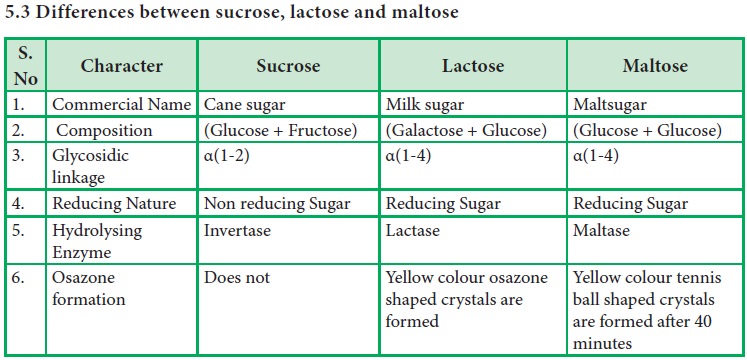
5.3
Differences between sucrose, lactose and maltose
Related Topics