Chapter: Modern Pharmacology with Clinical Applications: Drug Absorption and Distribution
Properties of Biological Membranes that Influence Drug Passage
PROPERTIES OF
BIOLOGICAL MEMBRANES THAT INFLUENCE DRUG PASSAGE
Although some substances are
translocated by special-ized transport mechanisms and small polar compounds may
filter through membrane pores, most foreign com-pounds penetrate cells by
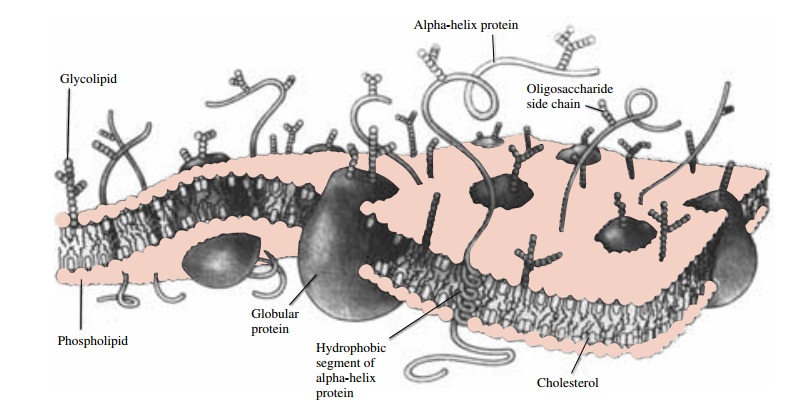
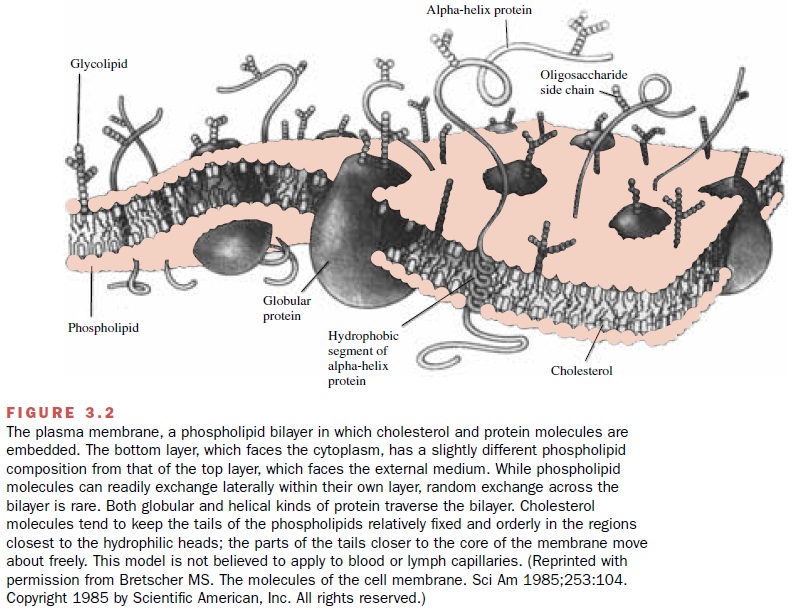
diffusing through lipid mem-branes. A model of membrane structure, shown in
Figure 3.2, envisions the membrane as a mosaic struc-ture composed of a
discontinuous bimolecular lipid layer
with fluidlike properties. A smaller component consists of glycoproteins or
lipoproteins that are em-bedded in the lipid matrix and have ionic and polar
groups protruding from one or both sides of the mem-brane. This membrane is
thought to be capable of un-dergoing rapid local shifts, whereby the relative
geome- try of specific adjacent proteins may change to form channels, or pores.
The pores permit the membrane to be less restrictive to the passage of
low-molecular-weight hydrophilic substances into cells. In addition to its role
as a barrier to solutes, the cell membrane has an important function in
providing a structural matrix for a variety of enzymes and drug receptors. The
model de-picted is not thought to
apply to capillaries.
Physicochemical Properties of Drugs and the Influence of pH
The ability of a drug to
diffuse across membranes is fre-quently expressed in terms of its lipid–water
partition co-efficient rather than its lipid solubility per se. This
coeffi-cient is defined as the ratio of the concentration of the drug in two
immiscible phases: a nonpolar liquid or or-ganic solvent (frequently octanol),
representing the mem-brane; and an aqueous buffer, usually at pH 7.4,
repre-senting the plasma. The partition coefficient is a measure of the
relative affinity of a drug for the lipid and aqueous phases. Increasing the
polarity of a drug, either by in-creasing its degree of ionization or by adding
a carboxyl, hydroxyl, or amino group to the molecule, decreases the lipid–water
partition coefficient. Alternatively, reducing drug polarity through
suppression of ionization or adding lipophilic (e.g., phenyl or t-butyl) groups
results in an in-crease in the lipid–water partition coefficient
Drugs, like most organic electrolytes, generally do not completely dissociate (i.e., form ions) in aqueous so-lution. Only a certain proportion of an organic drug molecule will ionize at a given pH. The smaller the frac-tion of total drug molecules ionized, the weaker the electrolyte. Since most drugs are either weak organic acids or bases (i.e., weak electrolytes), their degree of ionization will influence their lipid–water partition co-efficient and hence their ability to diffuse through mem-branes.
The proportion of the total
drug concentration that is present in either ionized or un-ionized form is
dic-tated by the drug’s dissociation or ionization constant (K) and the local
pH of the solution in which the drug is dissolved.
The dissociation of a weak
acid, RH, and a weak base, B, is described by the following equations:
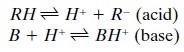
If these equations are
rewritten in terms of their dis-sociation constants (using Ka for
both weak acids and weak bases), we obtain
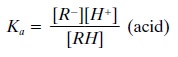
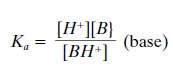
By taking logarithms and then
substituting the terms pK and pH for the negative logarithms of Ka
and [H+ ], respectively, we arrive at the Henderson-Hasselbach
equations:
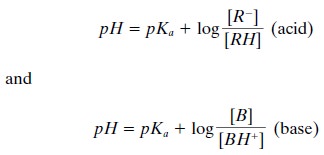
It is customary to describe
the dissociation constants of both
acids and bases in terms of pKa
values. This is possible in aqueous biological systems because a simple
mathematical relationship exists between pKa,
pKb, and the dissociation constant of water pKw
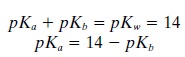
The use of only pKa values to describe the
relative strengths of either weak bases or weak acids makes comparisons between
drugs simpler. The lower the pKa
value (pKa< 6) of an
acidic drug, the stronger the acid (i.e., the larger the proportion of ionized
molecules). The higher the pKa
value (pKa > 8) of a
basic drug, the stronger the base. Thus, knowing the pH of the aqueous medium
in which the drug is dissolved and the pKa
of the drug, one can, using the Henderson-Hasselbach equation, calculate the
relative proportions of ionized and un-ionized drug present in solution. For
example, when the pKa of
the drug (e.g., 7) is the same as the pH (e.g., 7) of the surrounding medium,
there will be equal proportions of ionized [R-1 and un-ionized [RH]
mole-cules; that is, 50% of the drug is ionized.
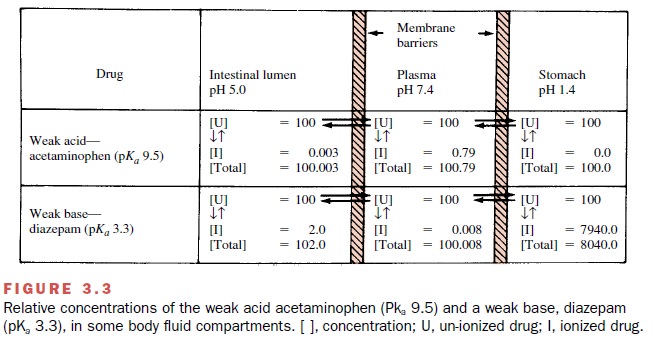
The effect of pH on drug
ionization is shown in Figure 3.3. The relationship between pH and degree of
drug ion-ization is not linear but sigmoidal; that is, small changes in pH may
greatly influence the degree of drug ionization, especially when pH and pKa values are initially
similar.
Related Topics