Chapter: Modern Pharmacology with Clinical Applications: Drug Absorption and Distribution
Factors Affecting Rate of Gastrointestinal Absorption
FACTORS
AFFECTING RATE OF GASTROINTESTINAL ABSORPTION
In addition to the
lipid–water partition coefficient of drugs, local blood flow, and intestinal
surface area, other factors may affect absorption from the gastrointestinal
tract.
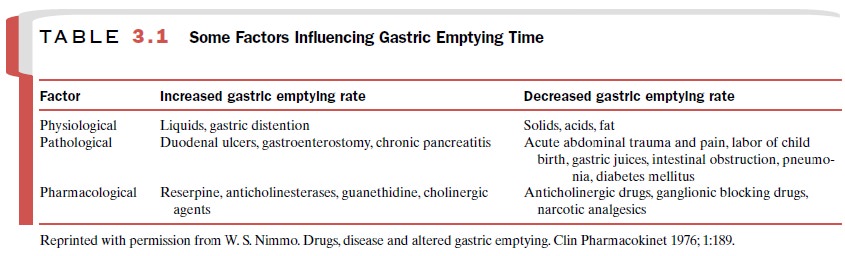
Gastric Emptying Time
The rate of gastric emptying
markedly influences the rate at which drugs are absorbed, whether they are
acids, bases, or neutral substances. In general, fac-tors that accelerate
gastric emptying time, thus permit-ting drugs to reach the large absorptive
surface of the small intestine sooner, will increase drug absorption unless the
drug is slow to dissolve. A list of physiolog-ical, pathological, and
pharmacological factors that in- fluence the rate of gastric emptying is
provided in Table 3.1.
Intestinal Motility
Increased gastrointestinal
motility may facilitate drug absorption by thoroughly mixing intestinal
contents and thereby bringing the drug into more intimate con-tact with the
mucosal surface. However, the opposite may also occur in that an increase in
motility may re-duce contact time in the upper portion of the intestine where
most of drug absorption occurs. Conversely, a decrease in gastrointestinal
motility may promote ab-sorption by increasing contact time. Thus, the effect
de-pends on the drug and change in motility. Serious in-testinal diseases,
particularly those associated with intestinal sloughing, can be expected to
alter drug ab-sorption dramatically.
Food
Absorption of most drugs from
the gastrointestinal tract is reduced or delayed by the presence of food in the
gut. Drugs such as the tetracyclines, which are highly ionized, can complex
with Ca++ ions in mem-branes, food, or milk, leading to a reduction
in their rate of absorption. For drugs that are ionized in the stomach and
un-ionized in the intestine, overall ab-sorption will be delayed by any factor
that delays gas-tric emptying. Finally, increased splanchnic blood flow, as
occurs during eating, will increase the rate of drug absorption.
Formulation Factors
The ability of solid drug
forms to dissolve and the sol-ubility of the individual drug in the highly
acidic gas-tric juice must be considered. For example, although the
anticoagulant dicumarol has a very high lipid– water partition coefficient, it
precipitates at the low pH of gastric juice, and the rate of its absorption is
thereby reduced. This may be overcome by covering the tablets with an enteric
coating that dissolves only in the relatively alkaline secretions in the small
intes-tine. Drugs administered in aqueous solution are absorbed faster and more
completely than tablet or suspension forms. Suspensions of fine particles
(mi-crocrystalline) are better absorbed than are those of larger particles.
Metabolism and Efflux Transporters
Drugs may be inactivated in
the gastrointestinal tract before they are absorbed. Until recently, only gut
mi-croflora were implicated in the metabolism of drugs in the gastrointestinal
system, affecting drug absorption. However, it has now become apparent that
drug-metabolizing enzymes, such as the cytochrome P450 en-zymes, play a major
role in determining the extent of drug absorption of some drugs. Significant
expression of cytochrome P450 3A4 and 3A5 occurs in the entero-cytes lining the
small intestine. These drug-metabolizing enzymes are responsible for
approximately 50% of the cytochrome P450–mediated drug metabolism and thus can be expected to play a major role
in the presystemic metabolism of a number of drugs. For example, less than 20%
of a dose of the im-munosuppressant cyclosporine reaches the systemic
cir-culation intact. In fact, most of the metabolism of cy-closporine prior to
reaching the systemic circulation takes place in the gut via cytochrome P450
3A4 and 3A5, not in the liver, as might be expected. Thus, gut me-tabolism is
the major factor responsible for the low per-centage of an oral dose of
cyclosporine reaching the sys-temic circulation. Cytochrome P450 2C9 and 2C19
are also expressed in measurable quantities in the human intestine. With any of
these four cytochrome P450 en-zymes, the variation in expression between
individuals is substantial, and so their relative contribution to presystemic
metabolism of drugs will vary from person to person.
Recently, it has also been
discovered that efflux transporters (transporters that pump drug or substrate
out of a cell) are also present in human intestinal ente-rocytes on the apical
side nearest the lumen of the in-testine. The predominant transporter protein
identified to date is P glycoprotein (Pgp), which is a product of the MDR1
gene. This transporter was originally identified as being overexpressed in
tumor cells and responsible in part for multidrug resistance because of its
role in the efflux of drugs out of tumor cells; thus the name mul-tidrug
resistance (MDR) gene. It has become apparent that many of the drugs that are
substrates for cy-tochrome P450 3A4 are also substrates for Pgp. As a substrate
for Pgp, a drug will enter the cell, usually via passive diffusion, but then be
picked up by the Pgp transporter and carried back to the gut lumen (efflux). As
this continually occurs along the intestine, some of the drug molecules are
prevented from being absorbed, which decreases overall absorption. Taken
together, the Pgp transporter and the cytochrome P450 enzymes form a mechanism
to reduce the amount of drug reach-ing the systemic circulation.
Related Topics